TOTAL ADVANCED HEALTH FRESH MINT- cetylpyridinium chloride rinse
Colgate-Palmolive Company
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Total Advanced Health Fresh Mint
Uses
- helps prevent and reduce plaque and gingivitis
- helps control plaque bacteria that contribute to the development of gingivitis and bleeding gums
Warnings
Directions
Shake well to mix the two liquids before each use.
| adults and children 12 years of age and older | rinse for 30 seconds with 20 mL (4 teaspoonfuls) twice a day. Do not swallow. |
| children 6 years to under 12 years of age | supervise use |
| children under 6 years of age | do not use |
Other information
- this rinse is not intended to replace brushing or flossing
- store at 59°F to 77°F
- keep from freezing
Inactive ingredients
water, mineral oil, glycerin, sorbitol, flavor, benzyl alcohol, potassium sorbate, sodium benzoate, phosphoric acid, sodium saccharin, sucralose, simethicone, helianthus annuus (sunflower) seed oil, beta carotene, FD&C blue no. 1
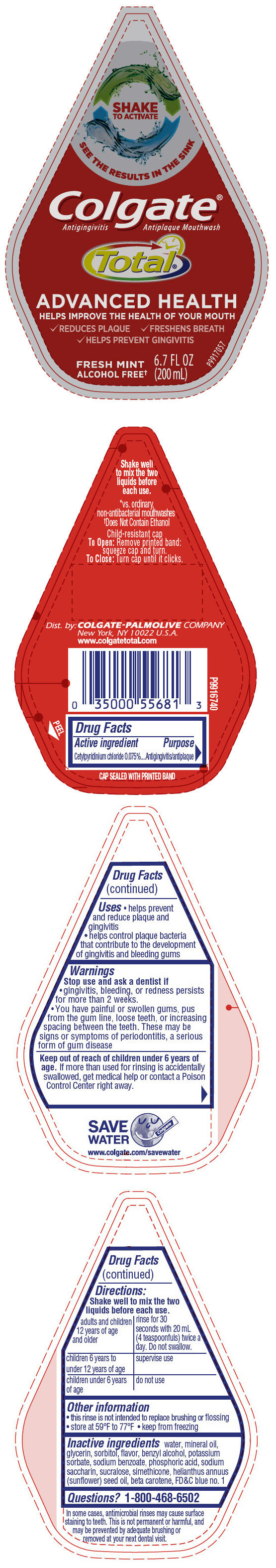
PRINCIPAL DISPLAY PANEL - 200 mL Bottle Label
SHAKE
TO ACTIVATE
SEE THE RESULTS IN THE SINK
Colgate®
Antigingivitis Antiplaque Mouthwash
Total®
ADVANCED HEALTH
HELPS IMPROVE THE HEALTH OF YOUR MOUTH
- ✓
- REDUCES PLAQUE
- ✓
- FRESHENS BREATH
- ✓
- HELPS PREVENT GINGIVITIS
FRESH MINT
ALCOHOL FREE†
6.7 FL OZ
(200 mL)
P9917057
| TOTAL ADVANCED HEALTH FRESH MINT
cetylpyridinium chloride rinse |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - Colgate-Palmolive Company (001344381) |