Label: FLUAID- menthol granule, for solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 62003-002-30 - Packager: PRINCE OF PEACE ENTERPRISES INC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 1, 2014
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- WHEN USING
- ASK DOCTOR/PHARMACIST
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- DO NOT USE
- WARNINGS
- QUESTIONS
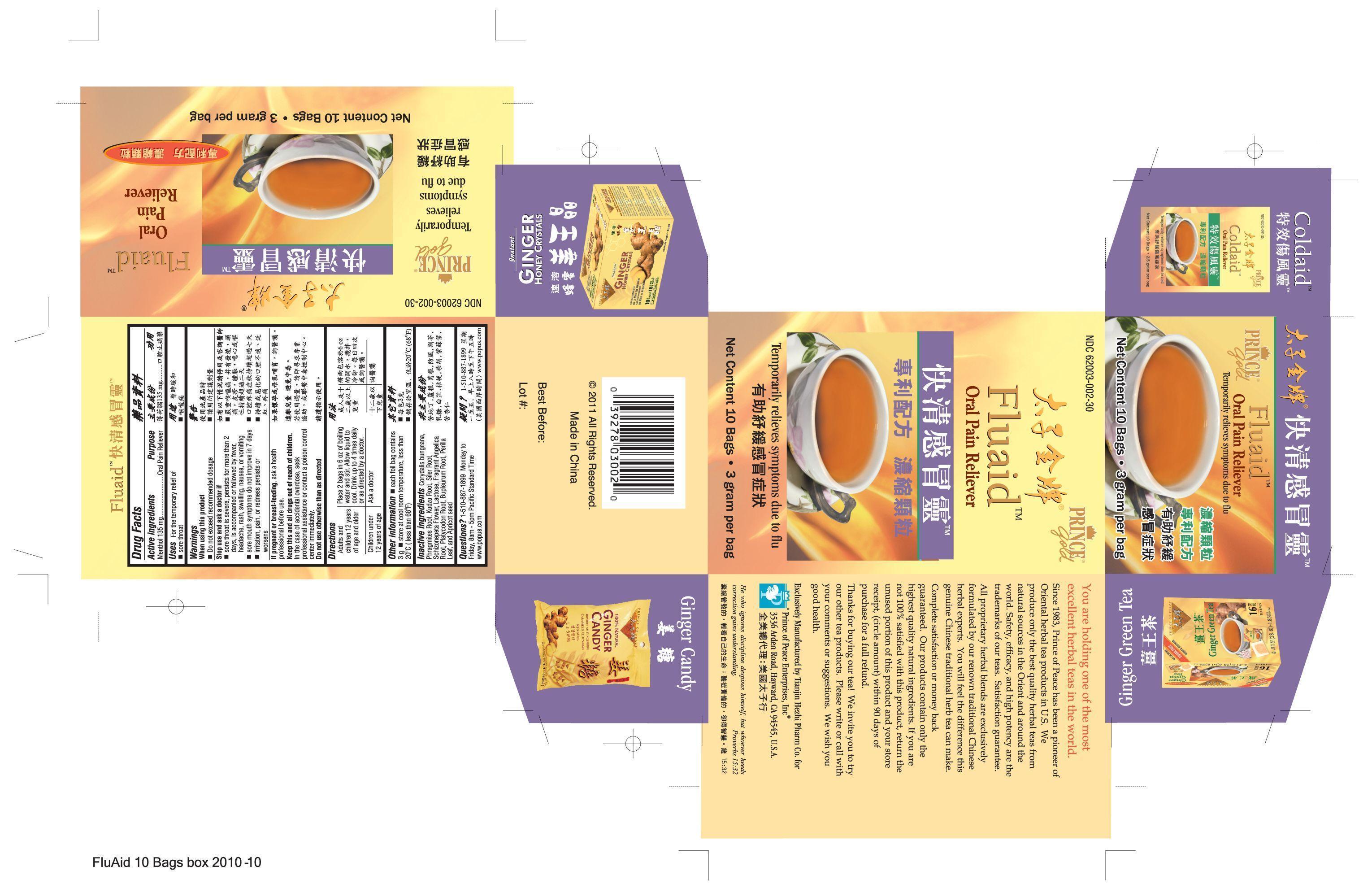
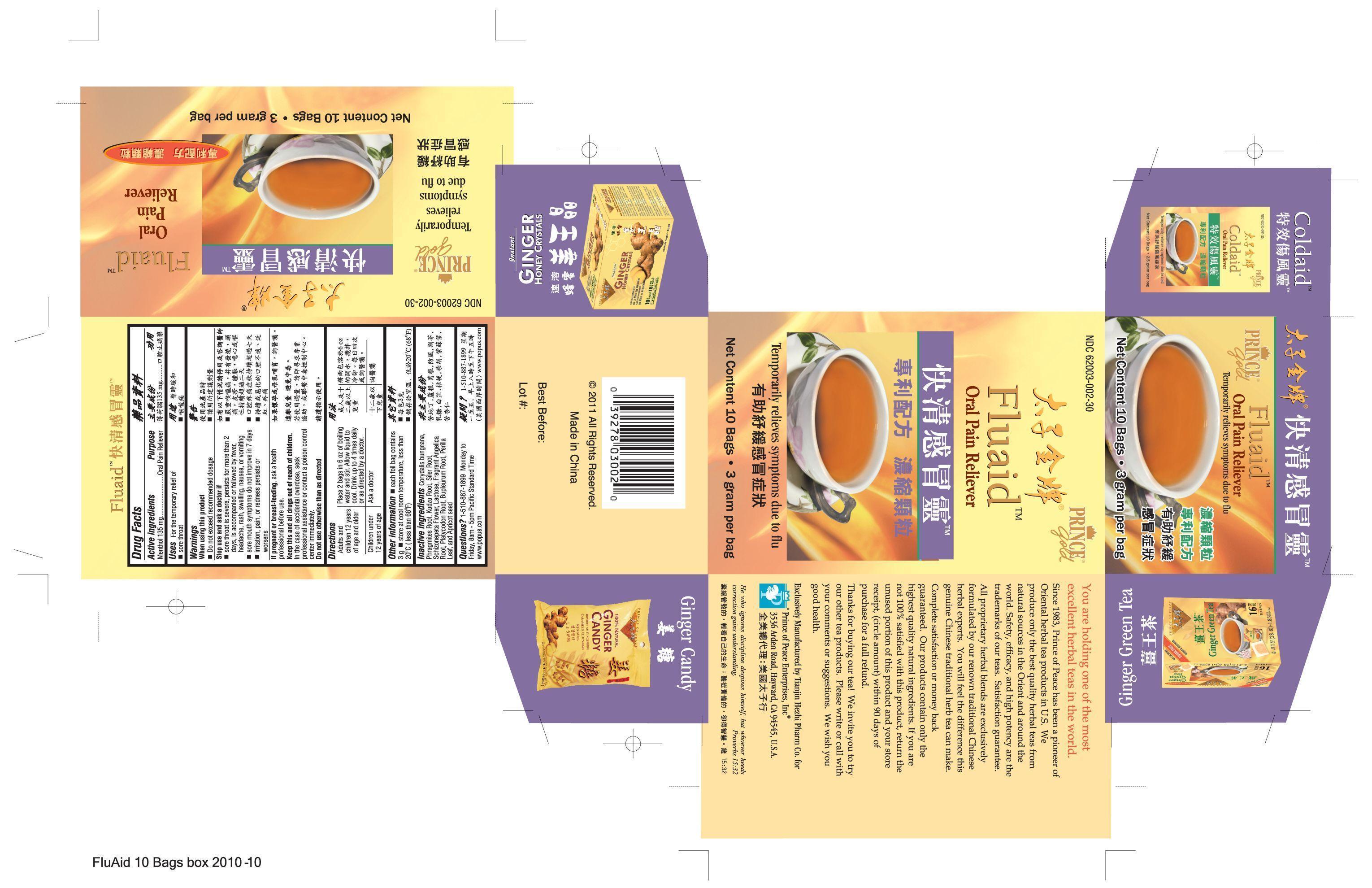
- PRINCIPAL DISPLAY PANEL
- INDICATIONS & USAGE
- DOSAGE & ADMINISTRATION
- INACTIVE INGREDIENT
-
INGREDIENTS AND APPEARANCE
FLUAID
menthol granule, for solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62003-002 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Menthol (UNII: L7T10EIP3A) (Menthol - UNII:L7T10EIP3A) Menthol 270 mg in 6 g Inactive Ingredients Ingredient Name Strength Angelica dahurica root (UNII: 1V63N2S972) Lactose (UNII: J2B2A4N98G) Bupleurum chinense root (UNII: BMA22623YK) APRICOT (UNII: 269CJD5GZ9) PUERARIA MONTANA VAR. CHINENSIS ROOT (UNII: FQN0D1U235) Saposhnikovia divaricata root (UNII: 8H84LFK2QD) SCHIZONEPETA TENUFOLIA SPIKE (UNII: 2FN3BA1MZE) PLATYCODON GRANDIFLORUM LEAF (UNII: 2L64H2X8DY) Perilla frutescens leaf (UNII: T4L5881Y68) CORYDALIS BUNGEANA FLOWERING/FRUITING TOP (UNII: 93W3R296YH) PHRAGMITES AUSTRALIS ROOT (UNII: BBJ7AOM815) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62003-002-30 10 in 1 BOX 1 3 g in 1 PACKET Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part356 12/01/2010 Labeler - PRINCE OF PEACE ENTERPRISES INC. (131132714)