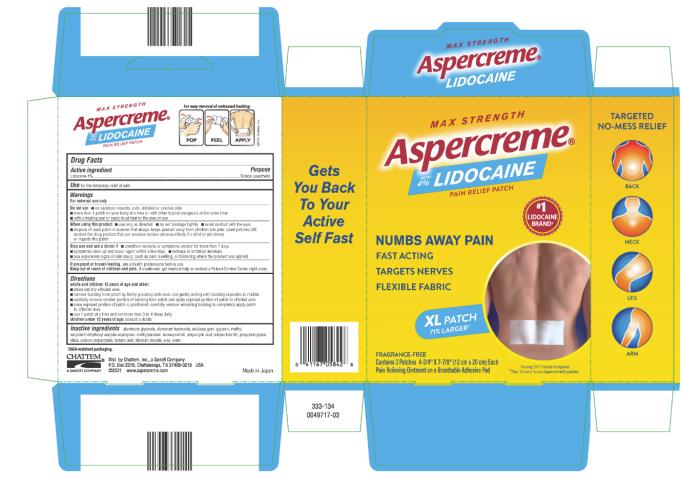
Label: ASPERCREME WITH LIDOCAINE XL- lidocaine patch
- NDC Code(s): 41167-0585-0, 41167-0585-1
- Packager: Chattem, Inc.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated October 25, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
- Uses
-
Warnings
For external use only
Do not use
- on puncture wounds, cuts, irritated or swollen skin
- more than 1 patch on your body at a time or with other topical analgesics at the same time
- with a heating pad or apply local heat to the area of use
When using this product
- use only as directed
- do not bandage tightly
- avoid contact with the eyes
- dispose of used patch in manner that always keeps product away from children and pets. Used patches still contain the drug product that can produce serious adverse effects if a child or pet chews or ingests this patch.
- on puncture wounds, cuts, irritated or swollen skin
-
Directions
adults and children over 12 years:
- clean and dry affected area
- remove backing from patch by firmly grasping both ends and gently pulling until backing separates in middle
- carefully remove smaller portion of backing from patch and apply exposed portion of patch to affected area
- once exposed portion of patch is positioned, carefully remove remaining backing to completely apply patch to affected area
- use 1 patch at a time and not more than 3 to 4 times daily
children 12 years or younger: consult a doctor
- clean and dry affected area
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ASPERCREME WITH LIDOCAINE XL
lidocaine patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:41167-0585 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 422 mg Inactive Ingredients Ingredient Name Strength DIHYDROXYALUMINUM AMINOACETATE ANHYDROUS (UNII: 1K713C615K) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) GLYCERIN (UNII: PDC6A3C0OX) METHYLPARABEN (UNII: A2I8C7HI9T) NONOXYNOL-30 (UNII: JJX07DG188) POLYGALIC ACID (UNII: XCC6WEA55L) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM POLYACRYLATE (8000 MW) (UNII: 285CYO341L) TARTARIC ACID (UNII: W4888I119H) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) UREA (UNII: 8W8T17847W) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:41167-0585-1 4 in 1 CARTON 10/21/2017 10/22/2017 1 1 in 1 POUCH; Type 0: Not a Combination Product 2 NDC:41167-0585-0 3 in 1 CARTON 10/21/2017 2 1 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 10/21/2017 Labeler - Chattem, Inc. (003336013)