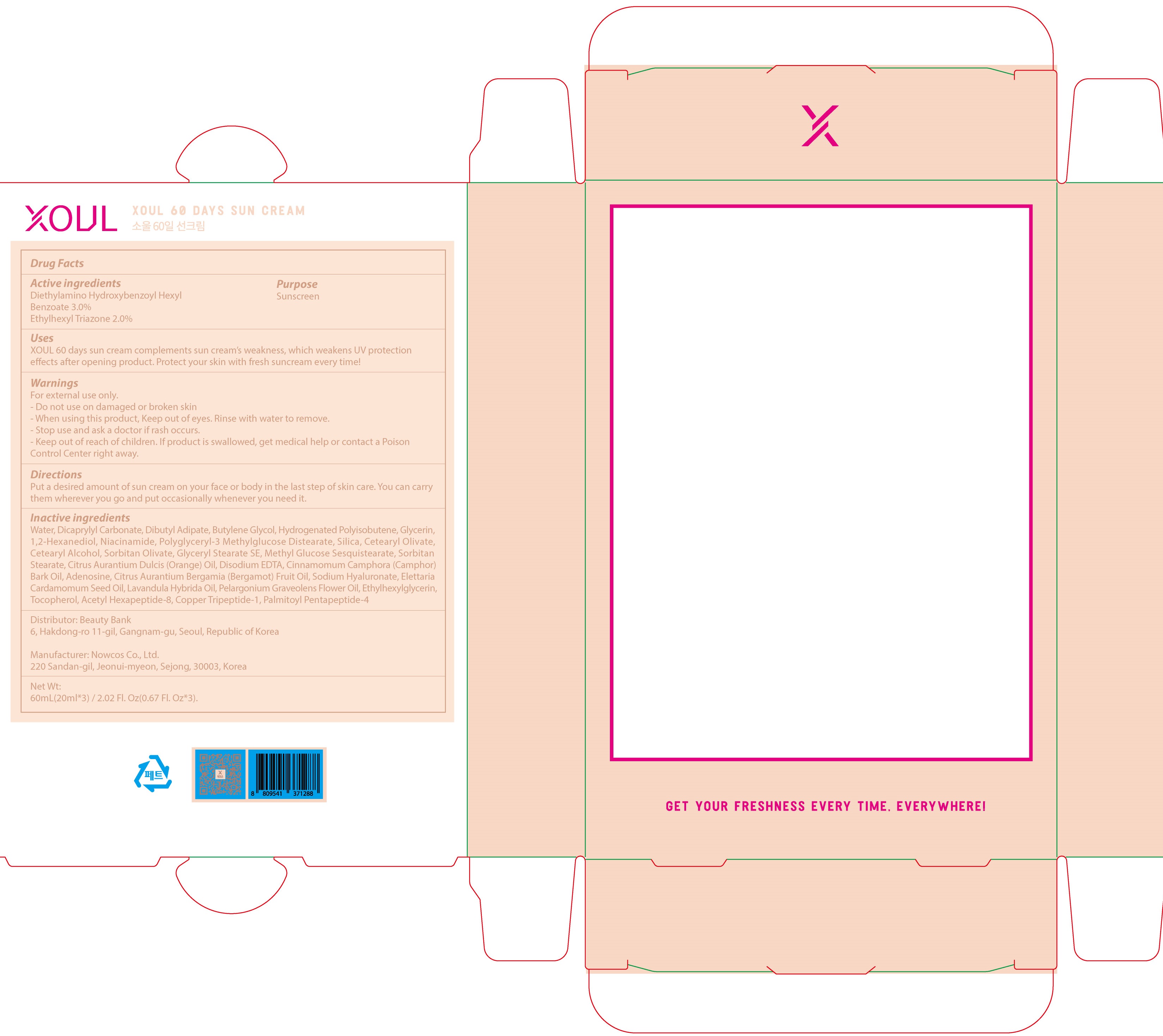
XOUL 60DAYS SUN- diethylamino hydroxybenzoyl hexyl benzoate, ethylhexyl triazone cream
CrossJ
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
ACTIVE INGREDIENT
Active ingredients: Diethylamino Hydroxybenzoyl Hexyl Benzoate 3.0%, Ethylhexyl Triazone 2.0%
INACTIVE INGREDIENT
Inactive ingredients:
Water, Dicaprylyl Carbonate, Dibutyl Adipate, Butylene Glycol, Hydrogenated Polyisobutene, Glycerin, 1,2-Hexanediol, Niacinamide, Polyglyceryl-3 Methylglucose Distearate, Silica, Cetearyl Olivate, Cetearyl Alcohol, Sorbitan Olivate, Glyceryl Stearate SE, Methyl Glucose Sesquistearate, Sorbitan Stearate, Citrus Aurantium Dulcis (Orange) Oil, Disodium EDTA, Cinnamomum Camphora (Camphor) Bark Oil, Adenosine, Citrus Aurantium Bergamia (Bergamot) Fruit Oil, Sodium Hyaluronate, Elettaria Cardamomum Seed Oil, Lavandula Hybrida Oil, Pelargonium Graveolens Flower Oil, Ethylhexylglycerin, Tocopherol, Acetyl Hexapeptide-8, Copper Tripeptide-1, Palmitoyl Pentapeptide-4
WARNINGS
Warnings:
For external use only.
- Do not use on damaged or broken skin
- When using this product, Keep out of eyes. Rinse with water to remove.
- Stop use and ask a doctor if rash occurs.
- Keep out of reach of children. If product is swallowed, get medical help or contact a Poison Control Center right away.
Uses
Uses:
XOUL 60 days sun cream complements sun cream’s weakness, which weakens UV protection effects after opening product. Protect your skin with fresh suncream every time!
| XOUL 60DAYS SUN
diethylamino hydroxybenzoyl hexyl benzoate, ethylhexyl triazone cream |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - CrossJ (694918159) |
| Registrant - CrossJ (694918159) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Nowcos.Co.,Ltd | 689914984 | manufacture(73695-020) | |