Label: ANTIPHLAMINE- l-menthol, l-camphor, methyl salicylate patch
- NDC Code(s): 73442-0010-1, 73442-0010-2
- Packager: I World Pharmaceutical Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 8, 2020
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
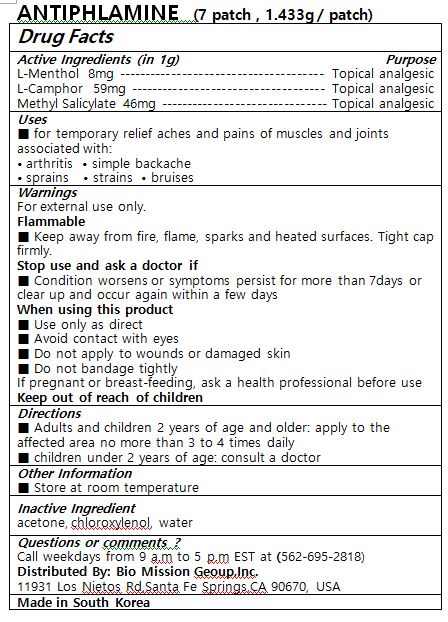
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
-
WARNINGS
Warnings
For external use only.
Flammable
■ Keep away from fire, flame, sparks and heated surfaces. Tight cap firmly.
Stop use and ask a doctor if
■ Condition worsens or symptoms persist for more than 7days or clear up and occur again within a few days
When using this product
■ Use only as direct
■ Avoid contact with eyes
■ Do not apply to wounds or damaged skin
■ Do not bandage tightly
If pregnant or breast-feeding, ask a health professional before use
Keep out of reach of children
- DOSAGE & ADMINISTRATION
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ANTIPHLAMINE
l-menthol, l-camphor, methyl salicylate patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73442-0010 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAMPHOR, (-)- (UNII: 213N3S8275) (CAMPHOR, (-)- - UNII:213N3S8275) CAMPHOR, (-)- 59 mg in 1 g LEVOMENTHOL (UNII: BZ1R15MTK7) (LEVOMENTHOL - UNII:BZ1R15MTK7) LEVOMENTHOL 8 mg in 1 g METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 46 mg in 1 g Inactive Ingredients Ingredient Name Strength ACETONE (UNII: 1364PS73AF) CHLOROXYLENOL (UNII: 0F32U78V2Q) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73442-0010-2 7 in 1 POUCH 09/09/2020 1 NDC:73442-0010-1 1.433 g in 1 PATCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 09/09/2020 Labeler - I World Pharmaceutical Co., Ltd. (688222857) Registrant - I World Pharmaceutical Co., Ltd. (688222857) Establishment Name Address ID/FEI Business Operations I World Pharmaceutical Co., Ltd. 688222857 manufacture(73442-0010)