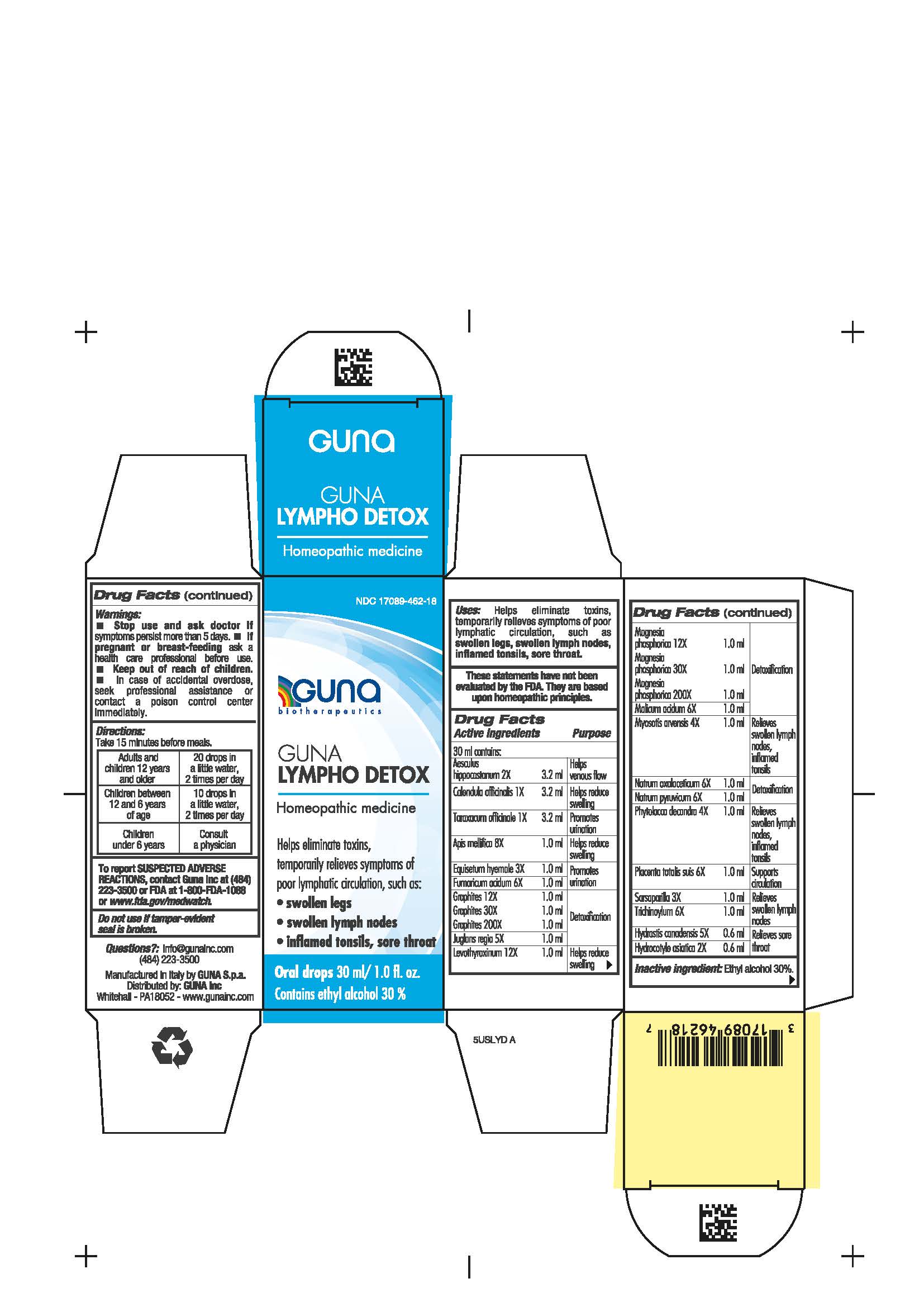
Label: GUNA LYMPHO DETOX- aesculus hippocastanum - apis mellifera - calendula officinalis flower - hexaketocyclohexane - equisetum hyemale - fumaric acid - graphite - hydrastis canadensis - hydrocotyle asiatica - juglans regia - levothyroxine - magnesium phosphate - malic acid - myosotis arvensis - phytolacca americana root - sarsaparilla - sodium diethyl oxalacetate - sodium pyruvate - sus scrofa placenta - taraxacum officinale - solution/ drops
- NDC Code(s): 17089-462-18
- Packager: Guna spa
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated October 23, 2020
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DIRECTIONS
-
ACTIVE INGREDIENTS/PURPOSE
AESCULUS HIPPOCASTANUM 2X HELPS VENOUS FLOW
CALENDULA OFFICINALIS 1X HELPS REDUCE SWELLING
TARAXACUM OFFICINALE 1X PROMOTES URINATION
APIS MELLIFICA 8X HELPS REDUCE SWELLING
EQUISETUM HYEMALE 3X PROMOTES URINATION
FUMARICUM ACIDUM 6X PROMOTES URINATION
GRAPHITES 12X 30X 200X DETOXIFICATION
JUGLANS REGIA 5X DETOXIFICATION
LEVOTHYROXINUM 12X HELPS REDUCE SWELLING
MAGNESIA PHOSPHORICA 12X 30X 200X DETOXIFICATION
MALICUM ACIDUM 6X DETOXIFICATION
MYOSOTIS ARVENSIS 4X RELIEVES SWOLLEN LYMPH NODES, INFLAMED TONSILS
NATRUM OXALACETICUM 6X DETOXIFICATION
NATRUM PYRUVICUM 6X DETOXIFICATION
PHYTOLACCA DECANDRA 4X RELIEVES SWOLLEN LYMPH NODES, INFLAMED TONSILS
PLACENTA TOTALIS SUIS 6X SUPPORTS CIRCULATION
SARSAPARILLA 3X RELIEVES SWOLLEN LYMPH NODES
TRICHINOYL PHOSPHATE 6X RELIEVES SWOLLEN LYMPH NODES
HYDRASTIS CANADENSIS 5X RELIEVES SORE THROAT
HYDROCOTYLE ASIATICA 2X RELIEVES SORE THROAT
- USES
- WARNINGS
- PREGNANCY
- WARNINGS
- QUESTIONS
- INDICATIONS & USAGE
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GUNA LYMPHO DETOX
aesculus hippocastanum - apis mellifera - calendula officinalis flower - hexaketocyclohexane - equisetum hyemale - fumaric acid - graphite - hydrastis canadensis - hydrocotyle asiatica - juglans regia - levothyroxine - magnesium phosphate - malic acid - myosotis arvensis - phytolacca americana root - sarsaparilla - sodium diethyl oxalacetate - sodium pyruvate - sus scrofa placenta - taraxacum officinale - solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:17089-462 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength APIS MELLIFERA (UNII: 7S82P3R43Z) (APIS MELLIFERA - UNII:7S82P3R43Z) APIS MELLIFERA 8 [hp_X] in 30 mL CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) (CALENDULA OFFICINALIS FLOWER - UNII:P0M7O4Y7YD) CALENDULA OFFICINALIS FLOWER 1 [hp_X] in 30 mL MALIC ACID (UNII: 817L1N4CKP) (MALIC ACID - UNII:817L1N4CKP) MALIC ACID 6 [hp_X] in 30 mL EQUISETUM HYEMALE (UNII: 59677RXH25) (EQUISETUM HYEMALE - UNII:59677RXH25) EQUISETUM HYEMALE 3 [hp_X] in 30 mL FUMARIC ACID (UNII: 88XHZ13131) (FUMARIC ACID - UNII:88XHZ13131) FUMARIC ACID 6 [hp_X] in 30 mL GRAPHITE (UNII: 4QQN74LH4O) (GRAPHITE - UNII:4QQN74LH4O) GRAPHITE 12 [hp_X] in 30 mL JUGLANS REGIA FRUIT RIND, IMMATURE (UNII: ZPS7Q5U53K) (JUGLANS REGIA FRUIT RIND, IMMATURE - UNII:ZPS7Q5U53K) JUGLANS REGIA FRUIT RIND, IMMATURE 5 [hp_X] in 30 mL LEVOTHYROXINE (UNII: Q51BO43MG4) (LEVOTHYROXINE - UNII:Q51BO43MG4) LEVOTHYROXINE 12 [hp_X] in 30 mL MAGNESIUM PHOSPHATE, DIBASIC TRIHYDRATE (UNII: HF539G9L3Q) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM PHOSPHATE, DIBASIC TRIHYDRATE 12 [hp_X] in 30 mL MYOSOTIS ARVENSIS (UNII: C73BK97H5J) (MYOSOTIS ARVENSIS - UNII:C73BK97H5J) MYOSOTIS ARVENSIS 4 [hp_X] in 30 mL SODIUM DIETHYL OXALACETATE (UNII: 6CA025Y4FG) (DIETHYL OXALACETATE - UNII:15S56468G7) SODIUM DIETHYL OXALACETATE 6 [hp_X] in 30 mL SODIUM PYRUVATE (UNII: POD38AIF08) (PYRUVIC ACID - UNII:8558G7RUTR) SODIUM PYRUVATE 6 [hp_X] in 30 mL PHYTOLACCA AMERICANA ROOT (UNII: 11E6VI8VEG) (PHYTOLACCA AMERICANA ROOT - UNII:11E6VI8VEG) PHYTOLACCA AMERICANA ROOT 4 [hp_X] in 30 mL SARSAPARILLA (UNII: 2H1576D5WG) (SARSAPARILLA - UNII:2H1576D5WG) SARSAPARILLA 3 [hp_X] in 30 mL TARAXACUM OFFICINALE (UNII: 39981FM375) (TARAXACUM OFFICINALE - UNII:39981FM375) TARAXACUM OFFICINALE 1 [hp_X] in 30 mL DODECAHYDROXYCYCLOHEXANE DIHYDRATE (UNII: 5BWD2J7B4W) (DODECAHYDROXYCYCLOHEXANE - UNII:I1Z9VS3H64) DODECAHYDROXYCYCLOHEXANE DIHYDRATE 6 [hp_X] in 30 mL SUS SCROFA PLACENTA (UNII: C8CV8867O8) (SUS SCROFA PLACENTA - UNII:C8CV8867O8) SUS SCROFA PLACENTA 6 [hp_X] in 30 mL HORSE CHESTNUT (UNII: 3C18L6RJAZ) (HORSE CHESTNUT - UNII:3C18L6RJAZ) HORSE CHESTNUT 2 [hp_X] in 30 mL GOLDENSEAL (UNII: ZW3Z11D0JV) (GOLDENSEAL - UNII:ZW3Z11D0JV) GOLDENSEAL 5 [hp_X] in 30 mL CENTELLA ASIATICA WHOLE (UNII: 7M867G6T1U) (CENTELLA ASIATICA WHOLE - UNII:7M867G6T1U) CENTELLA ASIATICA WHOLE 2 [hp_X] in 30 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) 9 mL in 30 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17089-462-18 1 in 1 BOX 09/24/2020 1 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 09/24/2020 Labeler - Guna spa (430538264) Establishment Name Address ID/FEI Business Operations Guna spa 338587646 manufacture(17089-462)