MEKINIST- trametinib tablet, film coated
GlaxoSmithKline LLC
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use MEKINIST safely and effectively. See full prescribing information for MEKINIST.
MEKINIST (trametinib) tablets, for oral use Initial U.S. Approval: 2013 RECENT MAJOR CHANGESINDICATIONS AND USAGEMEKINIST is a kinase inhibitor indicated, as a single agent or in combination with dabrafenib, for the treatment of patients with unresectable or metastatic melanoma with BRAF V600E or V600K mutations as detected by an FDA-approved test. (1, 14.1) Limitation of use: MEKINIST is not indicated for treatment of patients who have received prior BRAF-inhibitor therapy. (1) DOSAGE AND ADMINISTRATIONDOSAGE FORMS AND STRENGTHSTablets: 0.5 mg and 2 mg. (3) CONTRAINDICATIONSNone. (4) WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONSSee 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling. Revised: 11/2015 |
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
MEKINIST® is indicated, as a single agent or in combination with dabrafenib, for the treatment of patients with unresectable or metastatic melanoma with BRAF V600E or V600K mutations, as detected by an FDA-approved test [see Clinical Studies (14.1)].
Limitation of use: MEKINIST is not indicated for treatment of patients who have received prior BRAF-inhibitor therapy [see Clinical Studies (14.2)].
2 DOSAGE AND ADMINISTRATION
2.1 Patient Selection
Select patients for treatment of unresectable or metastatic melanoma with MEKINIST based on the presence of BRAF V600E or V600K mutation in tumor specimens [see Clinical Studies (14.1)]. Information on FDA-approved tests for the detection of BRAF V600 mutations in melanoma is available at: http://www.fda.gov/CompanionDiagnostics.
2.2 Recommended Dosing
The recommended dosage regimen is MEKINIST 2 mg orally taken once daily at the same time each day as a single agent or with dabrafenib. Continue treatment until disease progression or unacceptable toxicity occurs.
Take MEKINIST at least 1 hour before or 2 hours after a meal [see Clinical Pharmacology (12.3)]. Do not take a missed dose of MEKINIST within 12 hours of the next dose of MEKINIST.
2.3 Dose Modifications
Review the Full Prescribing Information for dabrafenib for recommended dose modifications. Dose modifications are not recommended for MEKINIST when administered with dabrafenib for the following adverse reactions of dabrafenib: non-cutaneous malignancies and uveitis.
For New Primary Cutaneous Malignancies
No dose modifications are required.
|
Dose Reductions for MEKINIST |
|
|
First Dose Reduction |
1.5 mg orally once daily |
|
Second Dose Reduction |
1 mg orally once daily |
|
Subsequent Modification |
Permanently discontinue if unable to tolerate MEKINIST 1 mg orally once daily |
|
Severity of Adverse Reactiona |
MEKINISTb |
|
Febrile Drug Reaction |
|
|
Withhold MEKINIST until fever resolves. Then resume MEKINIST at same or lower dose level. |
|
Cutaneous |
|
|
Withhold MEKINIST for up to 3 weeks.
|
|
Cardiac |
|
|
|
|
Permanently discontinue MEKINIST. |
|
Venous Thromboembolism |
|
|
Withhold MEKINIST for up to 3 weeks.
|
|
Permanently discontinue MEKINIST. |
|
Ocular Toxicities |
|
|
Withhold MEKINIST for up to 3 weeks.
|
|
Permanently discontinue MEKINIST. |
|
Pulmonary |
|
|
Permanently discontinue MEKINIST. |
|
Other |
|
|
Withhold MEKINIST
|
|
Or
|
|
Permanently discontinue MEKINIST. |
aNational Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 4.0.
bSee Table 1 for recommended dose reductions of MEKINIST.
3 DOSAGE FORMS AND STRENGTHS
0.5 mg tablets: Yellow, modified oval, biconvex, film-coated tablets with ‘GS’ debossed on one face and ‘TFC’ on the opposing face.
2 mg tablets: Pink, round, biconvex, film-coated tablets with ‘GS’ debossed on one face and ‘HMJ’ on the opposing face.
5 WARNINGS AND PRECAUTIONS
Review the Full Prescribing Information for dabrafenib for information on the serious risks of dabrafenib prior to initiation of MEKINIST with dabrafenib.
5.1 New Primary Malignancies
New primary malignancies, cutaneous and non-cutaneous, can occur when MEKINIST is administered with dabrafenib.
Cutaneous Malignancies
In Trial 2, the incidence of basal cell carcinoma in patients receiving MEKINIST and dabrafenib was 3.3% (7/209) compared with 6% (13/211) in patients receiving single-agent dabrafenib. The median time to first diagnosis of basal cell carcinoma was 5.1 months (range: 2.8 to 23.9 months) in the MEKINIST plus dabrafenib arm and was 4.4 months (range: 29 days to 16.5 months) in the dabrafenib arm. Among the 7 patients receiving MEKINIST with dabrafenib who developed basal cell carcinoma, 2 (29%) experienced more than one occurrence (range: 1 to 3).
Cutaneous squamous cell carcinomas (cuSCC) and keratoacanthoma occurred in 3% of patients receiving MEKINIST and dabrafenib and 10% of patients receiving single-agent dabrafenib. The median time to first diagnosis of cuSCC was 7.3 months (range: 1.8 to 16.8 months) in the MEKINIST plus dabrafenib arm and was 2 months (range: 9 days to 20.9 months) in the dabrafenib arm.
New primary melanoma occurred in 0.5% (1/209) of patients receiving MEKINIST and dabrafenib and in 1.9% (4/211) of patients receiving dabrafenib alone.
Perform dermatologic evaluations prior to initiation of MEKINIST when used with dabrafenib, every 2 months while on therapy, and for up to 6 months following discontinuation of the combination. No dose modifications of MEKINIST are recommended in patients who develop new primary cutaneous malignancies.
Non-Cutaneous Malignancies
Based on its mechanism of action, dabrafenib may promote growth and development of malignancies with activation of RAS through mutation or other mechanisms [refer to the Full Prescribing Information for dabrafenib]. In Trial 2, non-cutaneous malignancies occurred in 1.4% (3/209) of patients receiving MEKINIST plus dabrafenib and in 2.8% (6/211) of patients receiving single-agent dabrafenib.
Monitor patients receiving MEKINIST and dabrafenib closely for signs or symptoms of non-cutaneous malignancies. No dose modification is required for MEKINIST in patients who develop non-cutaneous malignancies [see Dosage and Administration (2.3)].
5.2 Hemorrhage
Hemorrhages, including major hemorrhages defined as symptomatic bleeding in a critical area or organ, can occur with MEKINIST.
In Trial 2, the incidence of hemorrhagic events in patients receiving MEKINIST and dabrafenib was 19% (40/209) compared with 15% (32/211) of patients receiving dabrafenib alone. Gastrointestinal hemorrhage occurred in 6% (12/209) of patients receiving MEKINIST in combination with dabrafenib compared with 2.8% (6/211) of patients receiving single-agent dabrafenib. In Trial 2, 1.4% (3/209) of patients receiving MEKINIST and dabrafenib developed fatal intracranial hemorrhage compared with none of the patients receiving single-agent dabrafenib alone.
Permanently discontinue MEKINIST for all Grade 4 hemorrhagic events and for any Grade 3 hemorrhagic events that do not improve. Withhold MEKINIST for Grade 3 hemorrhagic events; if improved, resume at the next lower dose level.
5.3 Venous Thromboembolism
Venous thromboembolism can occur with MEKINIST.
In Trial 2, deep venous thrombosis (DVT) and pulmonary embolism (PE) occurred in 2.8% (6/209) of patients receiving MEKINIST and dabrafenib compared with 0.9% (2/211) of patients receiving single-agent dabrafenib.
Advise patients to immediately seek medical care if they develop symptoms of DVT or PE, such as shortness of breath, chest pain, or arm or leg swelling. Permanently discontinue MEKINIST for life threatening PE. Withhold MEKINIST for uncomplicated DVT and PE for up to 3 weeks; if improved, MEKINIST may be resumed at a lower dose level [see Dosage and Administration (2.3)].
5.4 Cardiomyopathy
Cardiomyopathy, including cardiac failure, can occur with MEKINIST.
In clinical trials of MEKINIST, all patients were required to have an echocardiogram at baseline to document normal LVEF and repeat echocardiograms at Week 4, Week 12, and every 12 weeks thereafter.
In Trial 1, cardiomyopathy [defined as cardiac failure, left ventricular dysfunction, or decreased left ventricular ejection fraction (LVEF)] occurred in 7% (14/211) of patients receiving MEKINIST; no chemotherapy-treated patient in Trial 1 developed cardiomyopathy. The median time to onset of cardiomyopathy in patients receiving MEKINIST was 2.1 months (range: 16 days to 5.1 months); cardiomyopathy was identified within the first month of receiving MEKINIST in five of these 14 patients. Four percent of patients in Trial 1 required discontinuation (4/211) and/or dose reduction (7/211) of MEKINIST. Cardiomyopathy resolved in 10 of these 14 (71%) patients.
Across clinical trials of MEKINIST as a single agent (N = 329), 11% of patients developed evidence of cardiomyopathy [decrease in LVEF below institutional lower limits of normal (LLN) with an absolute decrease in LVEF ≥10% below baseline] and 5% demonstrated a decrease in LVEF below institutional LLN with an absolute decrease in LVEF of ≥20% below baseline.
In Trial 2, evidence of cardiomyopathy (decrease in LVEF below the institutional LLN with an absolute decrease in LVEF ≥ 10% below baseline) occurred in 6% (12/206) of patients receiving MEKINIST and dabrafenib and in 2.9% (6/207) of patients receiving single-agent dabrafenib. The median time to onset of cardiomyopathy in patients receiving MEKINIST and dabrafenib was 8.2 months (range: 28 days to 24.9 months); cardiomyopathy was identified within the first month of receiving MEKINIST and dabrafenib in 2 of these 12 patients. In patients receiving MEKINIST and dabrafenib, cardiomyopathy resulted in dose interruption (4.4%), dose reduction (2.4%), and permanent discontinuation (1.5%) of MEKINIST. Cardiomyopathy resolved in 10 of 12 patients receiving MEKINIST and dabrafenib.
Assess LVEF by echocardiogram or multigated acquisition (MUGA) scan before initiation of MEKINIST as a single agent or with dabrafenib, one month after initiation, and then at 2- to 3-month intervals while on treatment. Withhold MEKINIST for up to 4 weeks if absolute LVEF value decreases by 10% from pretreatment values and is less than the lower limit of normal. For symptomatic cardiomyopathy or persistent, asymptomatic LV dysfunction of >20% from baseline that is below LLN that does not resolve within 4 weeks, permanently discontinue MEKINIST [see Dosage and Administration (2.3)].
5.5 Ocular Toxicities
Retinal Vein Occlusion (RVO)
Across all clinical trials with MEKINIST, the incidence of RVO was 0.2% (4/1,749). RVO may lead to macular edema, decreased visual function, neovascularization, and glaucoma.
Urgently (within 24 hours) perform ophthalmological evaluation for patient-reported loss of vision or other visual disturbances. Permanently discontinue MEKINIST in patients with documented RVO [see Dosage and Administration (2.3)].
Retinal Pigment Epithelial Detachment (RPED)
Retinal pigment epithelial detachment (RPED) can occur with MEKINIST administration. Retinal detachments may be bilateral and multifocal, occurring in the central macular region of the retina or elsewhere in the retina. In Trial 1 and Trial 2, routine monitoring of patients to detect asymptomatic RPED was not conducted; therefore, the true incidence of this finding is unknown.
Perform ophthalmological evaluation periodically and at any time a patient reports visual disturbances. Withhold MEKINIST if RPED is diagnosed. If resolution of the RPED is documented on repeat ophthalmological evaluation within 3 weeks, resume MEKINIST. Reduce the dose or discontinue MEKINIST if no improvement after 3 weeks [see Dosage and Administration (2.3)].
5.6 Interstitial Lung Disease
In clinical trials of single-agent MEKINIST (N = 329), ILD or pneumonitis occurred in 2% of patients. In Trial 1, 2.4% (5/211) of patients treated with MEKINIST developed ILD or pneumonitis; all five patients required hospitalization. The median time to first presentation of ILD or pneumonitis was 5.3 months (range: 2 to 5.7 months). In Trial 2, 1.0% (2/209) of patients receiving MEKINIST and dabrafenib developed pneumonitis compared with none of the patients receiving single-agent dabrafenib.
Withhold MEKINIST in patients presenting with new or progressive pulmonary symptoms and findings including cough, dyspnea, hypoxia, pleural effusion, or infiltrates, pending clinical investigations. Permanently discontinue MEKINIST for patients diagnosed with treatment-related ILD or pneumonitis [see Dosage and Administration (2.3)].
5.7 Serious Febrile Reactions
Serious febrile reactions and fever of any severity accompanied by hypotension, rigors or chills, dehydration, or renal failure, can occur when MEKINIST is administered with dabrafenib.
Fever (serious and non-serious) occurred in 57% (119/209) of patients receiving MEKINIST and dabrafenib and in 33% (69/211) of patients receiving dabrafenib alone. The median time to initial onset of fever was 1.2 months (range: 1 day to 23.5 months) with a median duration of fever of 3 days (range: 1 day to 1.7 months) on the MEKINIST plus dabrafenib arm compared with a median time to initial onset of fever of 20 days (range: 1 day to 22.9 months) and median duration of fever of 3 days (range: 1 day to 1.9 months) on the dabrafenib arm. Approximately one-half of the patients who received MEKINIST and dabrafenib and experienced pyrexia had three or more discrete episodes.
Across clinical trials of MEKINIST administered with dabrafenib, serious febrile reactions or fever of any severity complicated by severe rigors/chills hypotension, dehydration, renal failure, or syncope, occurred in 17% (93/559) of patients receiving MEKINIST and dabrafenib. Fever was complicated by severe chills/rigors in 0.4% (2/559), dehydration in 1.8% (10/559), renal failure in 0.5% (3/559), and syncope in 0.7% (4/559) of patients.
Withhold MEKINIST for fever higher than 104ºF or for serious febrile reactions or fever accompanied by hypotension, rigors or chills, dehydration, or renal failure, and evaluate for signs and symptoms of infection. Monitor serum creatinine and other evidence of renal function during and following severe pyrexia. Refer to Table 2 for recommended dose modifications for adverse reactions [see Dosage and Administration (2.3)]. Administer antipyretics as secondary prophylaxis when resuming MEKINIST if patient had a prior episode of severe febrile reaction or fever associated with complications. Administer corticosteroids (e.g., prednisone 10 mg daily) for at least 5 days for second or subsequent pyrexia if temperature does not return to baseline within 3 days of onset of pyrexia, or for pyrexia associated with complications such as dehydration, hypotension renal failure, or severe chills/rigors, and there is no evidence of active infection.
5.8 Serious Skin Toxicity
Serious skin toxicity can occur with MEKINIST.
In Trial 1, the overall incidence of any skin toxicity, the most common of which were rash, dermatitis acneiform rash, palmar-plantar erythrodysesthesia syndrome, and erythema, was 87% in patients receiving MEKINIST and 13% in chemotherapy-treated patients. Severe skin toxicity occurred in 12% of patients treated with MEKINIST. Skin toxicity requiring hospitalization occurred in 6% of patients treated with MEKINIST, most commonly for secondary infections of the skin requiring intravenous antibiotics or severe skin toxicity without secondary infection. In comparison, no patients treated with chemotherapy required hospitalization for severe skin toxicity or infections of the skin. The median time to initial onset of skin toxicity in patients treated with MEKINIST was 15 days (range: 1 day to 7.3 months) and median time to resolution of skin toxicity was 1.6 months (range: 1 day to 9.3 months). Reductions in the dose of MEKINIST were required in 12% and permanent discontinuation of MEKINIST was required in 1% of patients with skin toxicity.
In Trial 2, the overall incidence of any skin toxicity was 55% for patients receiving MEKINIST and dabrafenib compared with 55% for patients receiving single-agent dabrafenib. No serious or severe cases of skin toxicity occurred in patients treated with MEKINIST and dabrafenib. The median time to initial onset of skin toxicity for patients receiving MEKINIST with dabrafenib was 1.9 months (range: 1 day to 22.1 months) and median time to resolution of skin toxicity for patients receiving MEKINIST with dabrafenib was 1.2 months (range: 1 day to 24.4 months). Reductions in the dose of MEKINIST were required in 5% of patients receiving MEKINIST and dabrafenib and no patients required permanent discontinuation of MEKINIST for skin toxicity.
Across clinical trials of MEKINIST administered with dabrafenib (N = 559), serious skin toxicity occurred in 0.7% (4/559) of patients.
Withhold MEKINIST for intolerable or severe skin toxicity. Resume MEKINIST at reduced doses in patients with improvement or recovery from skin toxicity within 3 weeks [see Dosage and Administration (2.3)].
5.9 Hyperglycemia
Hyperglycemia requiring an increase in the dose of, or initiation of insulin or oral hypoglycemic agent therapy can occur when MEKINIST is administered with dabrafenib.
In Trial 2, 27% (4/15) of patients with a history of diabetes who received MEKINIST and dabrafenib and 13% (2/16) of patients with a history of diabetes who received single-agent dabrafenib required more intensive hypoglycemic therapy. Grade 3 and Grade 4 hyperglycemia based on laboratory values occurred in 5% (11/208) and 0.5% (1/208) of patients receiving MEKINIST and dabrafenib, respectively, compared with 4.3% (9/209) for Grade 3 hyperglycemia and no patients with Grade 4 hyperglycemia for patients receiving single-agent dabrafenib.
Monitor serum glucose levels upon initiation and as clinically appropriate when MEKINIST is administered with dabrafenib in patients with pre-existing diabetes or hyperglycemia.
5.10 Embryo-Fetal Toxicity
Based on findings from animal studies and its mechanism of action, MEKINIST can cause fetal harm when administered to a pregnant woman. Trametinib was embryotoxic and abortifacient in rabbits at doses greater than or equal to those resulting in exposures approximately 0.3 times the human exposure at the recommended clinical dose. If MEKINIST is used during pregnancy, or if the patient becomes pregnant while taking MEKINIST, advise the patient of the potential risk to a fetus [see Use in Specific Populations (8.1)].
Advise female patients of reproductive potential to use effective contraception during treatment with MEKINIST and for 4 months after treatment. Advise patients to contact their healthcare provider if they become pregnant, or if pregnancy is suspected, while taking MEKINIST [see Use in Specific Populations (8.1, 8.3)].
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in another section of the label:
- •
- New Primary Malignancies [see Warnings and Precautions (5.1)]
- •
- Hemorrhage [see Warnings and Precautions (5.2)]
- •
- Venous Thromboembolism [see Warnings and Precautions (5.3)]
- •
- Cardiomyopathy [see Warnings and Precautions (5.4)]
- •
- Ocular Toxicities [see Warnings and Precautions (5.5)]
- •
- Interstitial Lung Disease [see Warnings and Precautions (5.6)]
- •
- Serious Febrile Reactions [see Warnings and Precautions (5.7)]
- •
- Serious Skin Toxicity [see Warnings and Precautions (5.8)]
- •
- Hyperglycemia [see Warnings and Precautions (5.9)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described in the Warnings and Precautions section and below reflect exposure to MEKINIST as a single agent and in combination with dabrafenib.
MEKINIST Administered as a Single Agent
MEKINIST as a single agent was evaluated in 329 patients including 107 (33%) exposed for greater than or equal to 6 months and 30 (9%) exposed for greater than or equal to one year. MEKINIST as a single agent was studied in open-label, single-arm trials (N = 118) or in an open-label, randomized, active-controlled trial (N = 211). The median age was 54 years, 60% were male, >99% were White, and all patients had metastatic melanoma. All patients received 2 mg once-daily doses of MEKINIST.
Table 3 presents adverse reactions identified from analyses of Trial 1, a randomized, open-label trial of patients with BRAF V600E or V600K mutation-positive melanoma receiving MEKINIST (N = 211) 2 mg orally once daily or chemotherapy (N = 99) (either dacarbazine 1,000 mg/m2 every 3 weeks or paclitaxel 175 mg/m2 every 3 weeks) [see Clinical Studies (14.1)]. Patients with abnormal LVEF, history of acute coronary syndrome within 6 months, or current evidence of Class II or greater congestive heart failure (New York Heart Association) were excluded from Trial 1. The median duration of treatment with MEKINIST was 4.3 months. In Trial 1, 9% of patients receiving MEKINIST experienced adverse reactions resulting in permanent discontinuation of trial medication. The most common adverse reactions resulting in permanent discontinuation of MEKINIST were decreased left ventricular ejection fraction (LVEF), pneumonitis, renal failure, diarrhea, and rash. Adverse reactions led to dose reductions in 27% of patients treated with MEKINIST. Rash and decreased LVEF were the most common reasons cited for dose reductions of MEKINIST.
|
Adverse Reactions |
MEKINIST |
Chemotherapy |
||
|
N = 211 |
N = 99 |
|||
|
All Gradesa |
Grades 3 and 4b |
All Gradesa |
Grades 3 and 4b |
|
|
Skin and subcutaneous tissue disorders | ||||
|
57 |
8 |
10 |
0 |
|
19 |
<1 |
1 |
0 |
|
11 |
0 |
0 |
0 |
|
10 |
2 |
1 |
0 |
|
10 |
0 |
1 |
0 |
|
Gastrointestinal disorders | ||||
|
43 |
0 |
16 |
2 |
|
15 |
2 |
2 |
0 |
|
13 |
1 |
5 |
1 |
|
Vascular disorders | ||||
|
32 |
1 |
4 |
0 |
|
15 |
12 |
7 |
3 |
|
13 |
<1 |
0 |
0 |
aNational Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.
bGrade 4 adverse reactions limited to rash (n = 1) in trametinib arm and diarrhea (n = 1) in chemotherapy arm.
cIncludes stomatitis, aphthous stomatitis, mouth ulceration, and mucosal inflammation.
dIncludes abdominal pain, lower abdominal pain, upper abdominal pain, and abdominal tenderness.
eIncludes lymphedema, edema, and peripheral edema.
fIncludes epistaxis, gingival bleeding, hematochezia, rectal hemorrhage, melena, vaginal hemorrhage, hemorrhoidal hemorrhage, hematuria, and conjunctival hemorrhage.
Other clinically important adverse reactions observed in less than or equal to 10% of patients (N = 329) treated with MEKINIST were:
Cardiac Disorders: Bradycardia
Gastrointestinal Disorders: Dry mouth.
Infections and Infestations: Folliculitis, rash pustular, cellulitis.
Musculoskeletal and Connective Tissue Disorders: Rhabdomyolysis.
Nervous System Disorders: Dizziness, dysgeusia.
Ocular Disorders: Blurred vision, dry eye.
Table 4. Percent-Patient Incidence of Laboratory Abnormalities Occurring at a Higher Incidence in Patients Treated with MEKINIST in Trial 1 [Between-arm Difference of ≥5% (All Grades) or ≥2% (Grades 3 or 4)a]
|
Test |
MEKINIST |
Chemotherapy |
||
|
N = 211 |
N = 99 |
|||
|
All Grades |
Grades 3 and 4 |
All Grades |
Grades 3 and 4 |
|
|
Increased aspartate aminotransferase (AST) |
60 |
2 |
16 |
1 |
|
Hypoalbuminemia |
42 |
2 |
23 |
1 |
|
Increased alanine aminotransferase (ALT) |
39 |
3 |
20 |
3 |
|
Anemia |
38 |
2 |
26 |
3 |
|
Increased alkaline phosphatase |
24 |
2 |
18 |
3 |
aNo Grade 4 events were reported in either treatment arm.
MEKINIST Administered with Dabrafenib
The safety of MEKINIST, administered with dabrafenib, was evaluated in 559 patients with previously untreated, unresectable or metastatic, BRAF V600 mutation-positive melanoma who received Mekinist in two trials, Trial 2 (n = 209) multicenter, double-blind, randomized (1:1), active-controlled trial and Trial 3 (n = 350) a multicenter, open-label, randomized (1:1), active-controlled trial. In both trials, patients received MEKINIST 2 mg orally once daily and dabrafenib 150 mg orally twice daily until disease progression or unacceptable toxicity. The trials excluded patients with abnormal left ventricular ejection fraction, history of acute coronary syndrome within 6 months, history of Class II or greater congestive heart failure (New York Heart Association), history of RVO or RPED, QTcB interval ≥480 msec, uncontrolled hypertension, uncontrolled arrhythmias, active brain metastases, or known history of G6PD deficiency.
Among these 559 patients, 197 (35%) were exposed to MEKINIST for >6 months to 12 months while 185 (33%) were exposed to MEKINIST for >1 year. The median age was 55 years (range: 18 to 91), 57% were male, and 98% were White, 72% had baseline ECOG performance status 0 and 28% had ECOG performance status 1, 64% had M1c stage disease, 35% had elevated LDH at baseline, and 0.5% had a history of brain metastases.
The most commonly occurring adverse reactions (≥20%) for MEKINIST in patients receiving MEKINIST plus dabrafenib were: pyrexia, nausea, rash, chills, diarrhea, vomiting, hypertension, and peripheral edema.
The demographics and baseline tumor characteristics of patients enrolled in Trial 2 are summarized in Clinical Studies [see Clinical Studies (14.1)]. Patients receiving MEKINIST plus dabrafenib had a median duration of exposure of 11 months (range: 3 days to 30 months) to MEKINIST. Among the 209 patients receiving MEKINIST plus dabrafenib, 26% were exposed to MEKINIST for >6 months to 12 months while 46% were exposed to MEKINIST for >1 year.
In Trial 2, adverse reactions leading to discontinuation of MEKINIST occurred in 11% of patients receiving MEKINIST plus dabrafenib; the most common were pyrexia (1.4%) and decreased ejection fraction (1.4%). Adverse reactions leading to dose reductions of MEKINIST occurred in 18% of patients receiving MEKINIST plus dabrafenib; the most common were pyrexia (2.9%), neutropenia (1.9%), decreased ejection fraction (1.9%), and rash (1.9%). Adverse reactions leading to dose interruptions of MEKINIST occurred in 46% of patients receiving MEKINIST plus dabrafenib; the most common were pyrexia (18%), chills (7%), vomiting (6%) and decreased ejection fraction (4.8%).
Table 5 and Table 6 present selected adverse drug reactions and laboratory abnormalities, respectively, of MEKINIST observed in Trial 2.
Table 5. Incidence of Adverse Reactions Occurring in ≥10% (All Grades) of Patients Receiving MEKINIST with Dabrafenib and at a Higher Incidence* than in Patients Receiving Single-Agent Dabrafenib in Trial 2a
|
Adverse Reactions |
Pooled MEKINIST plus Dabrafenib N = 559 |
Trial 2 |
||||
|
MEKINIST plus Dabrafenib N =209 |
Dabrafenib N = 211 |
|||||
|
All Grades (%) |
Grades 3 and 4 (%) |
All Grades (%) |
Grades 3 and 4 (%) |
All Grades (%) |
Grades 3 and 4 (%) |
|
|
General disorders and administrative site conditions |
||||||
|
54 |
5 |
57 |
7 |
33 |
1.9 |
|
31 |
0.5 |
31 |
0 |
17 |
0.5 |
|
21 |
0.7 |
25 |
1.4 |
11 |
0.5 |
|
Gastrointestinal disorders |
||||||
|
35 |
0.4 |
34 |
0.5 |
27 |
1.4 |
|
31 |
1.3 |
30 |
1.4 |
16 |
0.9 |
|
27 |
1.1 |
25 |
1.0 |
14 |
0.5 |
|
18 |
0.9 |
26 |
1.0 |
14 |
2.4 |
|
Nervous system disorders |
||||||
|
11 |
0.2 |
14 |
0 |
7 |
0 |
|
Vascular disorders |
||||||
|
26 |
11 |
25 |
6 |
16 |
6 |
|
18 |
2.0 |
19 |
1.9 |
15 |
1.9 |
|
Skin and subcutaneous tissue disorders |
||||||
|
32 |
1.1 |
42 |
0 |
27 |
1.4 |
* ≥5% for All Grades or ≥2% for Grades 3–4 incidence in patients receiving MEKINIST with dabrafenib compared with patients receiving dabrafenib as a single agent
aNational Cancer Institute Common Terminology Criteria for Adverse Events, version 4.
bIncludes peripheral edema, edema, lymphedema, localized edema , and generalized edema.
cIncludes abdominal pain, upper abdominal pain, lower abdominal pain, and abdominal discomfort.
- dMost common events (≥1%) include epistaxis, hematochezia, decreased hemoglobin, purpura, and rectal hemorrhage. Grade 4 events were limited to hepatic hematoma and duodenal ulcer hemorrhage (each n = 1 in the pooled combination arm).
- eIncludes rash, generalized rash, pruritic rash, erythematous rash, papular rash, vesicular rash, macular rash, maculo-papular, and folliculitis rash.
Other clinically important adverse reactions for MEKINIST observed in less than 10% of patients receiving MEKINIST in combination with dabrafenib (N = 559) were:
Cardiac Disorders: bradycardia
Musculoskeletal Disorders: rhabdomyolysis
Table 6. Treatment-Emergent Laboratory Abnormalities Occurring at ≥10% (All Grades) of Patients Receiving MEKINIST with Dabrafenib and at a Higher Incidence* than in Patients Receiving Single-Agent Dabrafenib in Trial 2
|
Test |
Pooled MEKINIST plus Dabrafenib N = 559a |
Trial 2 |
||||
|
MEKINIST plus Dabrafenib N = 209b |
Dabrafenib N = 211b |
|||||
|
All Grades (%) |
Grades 3 and 4c (%) |
All Grades (%) |
Grades 3 and 4c (%) |
All Grades (%) |
Grades 3 and 4c (%) |
|
|
Hematology |
||||||
|
46 |
7 |
50 |
6 |
16 |
1.9 |
|
43 |
2.3 |
43 |
2.4 |
38 |
4.3 |
|
32 |
8 |
38 |
9 |
28 |
7 |
|
21 |
0.7 |
19 |
0.5 |
10 |
0.5 |
|
Liver Function Tests |
||||||
|
59 |
4.1 |
60 |
4.3 |
21 |
1.0 |
|
49 |
2.7 |
50 |
1.0 |
25 |
0.5 |
|
48 |
4.5 |
44 |
3.8 |
28 |
1.0 |
|
Chemistry |
||||||
|
60 |
4.7 |
65 |
6 |
57 |
4.3 |
|
48 |
1.1 |
53 |
1.4 |
27 |
0 |
|
25 |
8 |
24 |
6 |
14 |
2.9 |
* ≥5% for All Grades or ≥2% for Grades 3–4 incidence in patients receiving MEKINIST with dabrafenib compared with patients receiving dabrafenib as a single agent
AST = Aspartate aminotransferase; ALT = Alanine aminotransferase.
aFor these laboratory tests the denominator is 556.
bFor these laboratory tests the denominator is 208 for the combination arm, 207-209 for the dabrafenib arm.
c Grade 4 adverse reactions limited to lymphopenia and hyperglycemia (each n = 4), increased ALT and increased AST (each n = 3), neutropenia (n = 2), and hyponatremia (n = 1), in the pooled combination arm; neutropenia, lymphopenia, increased ALT, increased AST, hyperglycemia (each n = 1) in the Trial 2 combination arm; neutropenia, thrombocytopenia, increased ALT, and increased AST (each n = 1) in the dabrafenib arm.
7 DRUG INTERACTIONS
No formal clinical trials have been conducted to evaluate human cytochrome P450 (CYP) enzyme-mediated drug interactions with trametinib [see Clinical Pharmacology (12.3)].
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary:
Based on its mechanism of action and findings from animal reproduction studies, MEKINIST can cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology (12.1)]. There is insufficient data in pregnant women exposed to MEKINIST to assess the risks. Trametinib was embryotoxic and abortifacient in rabbits at doses greater than or equal to those resulting in exposures approximately 0.3 times the human exposure at the recommended clinical dose [see Data]. If MEKINIST is used during pregnancy, or if the patient becomes pregnant while taking MEKINIST, advise the patient of the potential risk to the fetus.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data:
Animal Data: In reproductive toxicity studies, administration of trametinib to rats during the period of organogenesis resulted in decreased fetal weights at doses greater than or equal to 0.031 mg/kg/day (approximately 0.3 times the human exposure based on AUC at the recommended dose). In rats, at a dose resulting in exposures 1.8-fold higher than the human exposure at the recommended dose, there was maternal toxicity and an increase in post-implantation loss.
In pregnant rabbits, administration of trametinib during the period of organogenesis resulted in decreased fetal body weight and increased incidence of variations in ossification at doses greater than or equal to 0.039 mg/kg/day (approximately 0.08 times the human exposure at the recommended dose based on AUC). In rabbits administered trametinib at 0.15 mg/kg/day (approximately 0.3 times the human exposure at the recommended dose based on AUC) there was an increase in post-implantation loss, including total loss of pregnancy, compared with control animals.
8.2 Lactation
Risk Summary
There are no data on the presence of trametinib in human milk, or the effects of trametinib on the breastfed infant, or on milk production. Because of the potential for serious adverse reactions in breastfed infants from MEKINIST, advise women not to breastfeed during treatment with MEKINIST and for 4 months following the last dose.
8.3 Females and Males of Reproductive Potential
Based on its mechanism of action and findings from animal reproduction studies, MEKINIST can cause fetal harm when administered to pregnant women [see Use in Specific Populations (8.1)].
Contraception
Females: Advise female patients of reproductive potential to use effective contraception during treatment with MEKINIST and for 4 months after the last dose. Advise patients to contact their healthcare provider if they become pregnant, or if pregnancy is suspected, while taking MEKINIST.
Infertility
Females: Advise female patients of reproductive potential that MEKINIST may impair fertility. Increased follicular cysts and decreased corpora lutea were observed in female rats at dose exposures equivalent to 0.3 times the human exposure at the recommended dose [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
The safety and effectiveness of MEKINIST as a single agent or in combination with dabrafenib have not been established in pediatric patients.
Juvenile Animal Data
In a repeat-dose toxicity study in juvenile rats, decreased bone length and corneal dystrophy were observed at doses resulting in exposures as low as 0.3 times the human exposure at the recommended adult dose based on AUC. Additionally, a delay in sexual maturation was noted at doses resulting in exposures as low as 1.6 times the human exposure at the recommended adult dose based on AUC.
8.5 Geriatric Use
Clinical trials of MEKINIST as a single agent did not include sufficient numbers of subjects aged 65 and older to determine whether they respond differently from younger subjects.
Of the 559 patients randomized to receive MEKINIST plus dabrafenib in clinical trials, 24% were aged 65 years and older and 6% patients aged 75 years and older. No overall differences in the effectiveness of MEKINIST plus dabrafenib were observed in elderly patients as compared to younger patients. The incidences of peripheral edema (26% vs. 12%) and anorexia (21% vs. 9%) increased in elderly patients as compared to younger patients.
8.6 Hepatic Impairment
No dedicated clinical trial has been conducted to evaluate the effect of hepatic impairment on the pharmacokinetics of trametinib. No dose adjustment is recommended in patients with mild hepatic impairment based on a population pharmacokinetic analysis [see Clinical Pharmacology (12.3)].
The appropriate dose of MEKINIST has not been established in patients with moderate or severe hepatic impairment.
8.7 Renal Impairment
No formal clinical trial has been conducted to evaluate the effect of renal impairment on the pharmacokinetics of trametinib. No dose adjustment is recommended in patients with mild or moderate renal impairment based on a population pharmacokinetic analysis [see Clinical Pharmacology (12.3)]. The appropriate dose of MEKINIST has not been established in patients with severe renal impairment.
10 OVERDOSAGE
The highest doses of MEKINIST evaluated in clinical trials were 4 mg orally once daily and 10 mg administered orally once daily on 2 consecutive days followed by 3 mg once daily. In seven patients treated on one of these two schedules, there were two cases of retinal pigment epithelial detachments for an incidence of 28%.
Since trametinib is highly bound to plasma proteins, hemodialysis is likely to be ineffective in the treatment of overdose with MEKINIST.
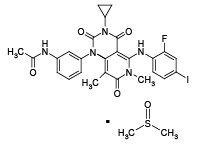
11 DESCRIPTION
Trametinib dimethyl sulfoxide is a kinase inhibitor. The chemical name is acetamide, N-[3-[3-cyclopropyl-5-[(2-fluoro-4- iodophenyl)amino]-3,4,6,7-tetrahydro-6,8-dimethyl- 2,4,7-trioxopyrido[4,3-d]pyrimidin-1(2H)-yl]phenyl]-, compound with 1,1’-sulfinylbis[methane] (1:1). It has a molecular formula C26H23FIN5O4•C2H6OS with a molecular mass of 693.53. Trametinib dimethyl sulfoxide has the following chemical structure:
Trametinib dimethyl sulfoxide is a white to almost white powder. It is practically insoluble in the pH range of 2 to 8 in aqueous media.
MEKINIST (trametinib) tablets are supplied as 0.5 mg and 2 mg tablets for oral administration. Each 0.5 mg tablet contains 0.5635 mg trametinib dimethyl sulfoxide equivalent to 0.5 mg of trametinib non-solvated parent. Each 2 mg tablet contains 2.254 mg trametinib dimethyl sulfoxide equivalent to 2 mg of trametinib non-solvated parent.
The inactive ingredients of MEKINIST tablets are: Tablet Core: colloidal silicon dioxide, croscarmellose sodium, hypromellose, magnesium stearate (vegetable source), mannitol, microcrystalline cellulose, sodium lauryl sulfate. Coating: hypromellose, iron oxide red (2 mg tablets), iron oxide yellow (0.5 mg tablets), polyethylene glycol, polysorbate 80 (2 mg tablets), titanium dioxide.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Trametinib is a reversible inhibitor of mitogen-activated extracellular signal-regulated kinase 1 (MEK1) and MEK2 activation and of MEK1 and MEK2 kinase activity. MEK proteins are upstream regulators of the extracellular signal-related kinase (ERK) pathway, which promotes cellular proliferation. BRAF V600E mutations result in constitutive activation of the BRAF pathway which includes MEK1 and MEK2. Trametinib inhibits BRAF V600 mutation-positive melanoma cell growthin vitro and in vivo.
Trametinib and dabrafenib target two different kinases in the RAS/RAF/MEK/ERK pathway. Use of trametinib and dabrafenib in combination resulted in greater growth inhibition of BRAF V600 mutation-positive melanoma cell lines in vitro and prolonged inhibition of tumor growth in BRAF V600 mutation positive melanoma xenograftscompared with either drug alone.
12.2 Pharmacodynamics
Administration of 1 mg and 2 mg MEKINIST to patients with BRAF V600 mutation-positive melanoma resulted in dose-dependent changes in tumor biomarkers including inhibition of phosphorylated ERK, inhibition of Ki67 (a marker of cell proliferation), and increases in p27 (a marker of apoptosis).
Cardiac Electrophysiology
The heart rate-corrected QT (QTc) prolongation potential of trametinib was assessed in a dedicated study in 32 patients who received placebo on day 1 and MEKINIST 2 mg once daily on days 2-14 followed by MEKINIST 3 mg on day 15. No clinically relevant QTc prolongation was detected in the study.
In clinical trials in patients receiving MEKINIST plus dabrafenib, QTc prolongation >500 ms occurred in 0.8% (2/264) of patients, and QTc increased by >60 ms from baseline in 3.8% (10/264) of patients.
12.3 Pharmacokinetics
The pharmacokinetics (PK) of trametinib were characterized following single- and repeat-oral administration in patients with solid tumors and BRAF V600 mutation-positive metastatic melanoma.
Absorption
After oral administration of MEKINIST, the median time to achieve peak plasma concentrations (Tmax) is 1.5 hours post-dose. The mean absolute bioavailability of a single 2 mg oral dose of MEKINIST is 72%. The increase in Cmax was dose proportional after a single dose of 0.125 to 10 mg while the increase in AUC was greater than dose proportional. After repeat doses of 0.125 to 4 mg daily, both Cmax and AUC increase proportionally with dose. Inter-subject variability in AUC and Cmax at steady state is 22% and 28%, respectively.
Administration of a single dose of MEKINIST with a high-fat, high-calorie meal decreased trametinib AUC by 24%, Cmax by 70%, and delayed Tmax by approximately 4 hours as compared with fasted conditions [see Dosage and Administration (2.2)].
Distribution
Trametinib is 97.4% bound to human plasma proteins. The apparent volume of distribution (Vc/F) is 214 L.
Metabolism
Trametinib is metabolized predominantly via deacetylation alone or with mono-oxygenation or in combination with glucuronidation biotransformation pathways in vitro. Deacetylation is mediated by carboxylesterases (i.e., carboxylesterase 1b/c and 2) and may also be mediated by other hydrolytic enzymes.
Following a single dose of [14C]-trametinib, approximately 50% of circulating radioactivity is represented as the parent compound. However, based on metabolite profiling after repeat dosing of trametinib, ≥75% of drug-related material in plasma is the parent compound.
Elimination
The estimated elimination half-life of trametinibbased on the population PK model is 3.9 to 4.8 days. The apparent clearance is 4.9 L/h.
Following oral administration of [14C]-trametinib, greater than 80% of excreted radioactivity was recovered in the feces while less than 20% of excreted radioactivity was recovered in the urine with less than 0.1% of the excreted dose as parent.
Specific Populations
Age, Body Weight, and Gender: Based on a population pharmacokinetic analysis, age, sex, and body weight do not have a clinically important effect on the exposure of trametinib. There are insufficient data to evaluate potential differences in the exposure of trametinib by race or ethnicity.
Hepatic Impairment: Based on a population pharmacokinetic analysis in 64 patients with mild hepatic impairment (total bilirubin ≤ULN and AST >ULN or total bilirubin greater than 1.0 to 1.5 x ULN and any AST), mild hepatic impairment has no clinically important effect on the systemic exposure of trametinib. The pharmacokinetics of trametinib have not been studied in patients with moderate or severe hepatic impairment [see Use in Specific Populations (8.6)].
Renal Impairment: As renal excretion of trametinib is low (less than 20%), renal impairment is unlikely to have a clinically important effect on the exposure of trametinib. Based on a population PK analysis in 223 patients with mild renal impairment (GFR 60 to 89 mL/min/1.73 m2) and 35 patients with moderate renal impairment (GFR 30 to 59 mL/min/1.73 m2), mild and moderate renal impairment have no clinically important effects on the systemic exposure of trametinib. The pharmacokinetics of trametinib have not been studied in patients with severe renal impairment [see Use in Specific Populations (8.7)].
Pediatrics: No trials have been conducted to evaluate the pharmacokinetics of trametinib in pediatric patients.
Drug Interactions
Effect of CYP Enzymes on Trametinib: Trametinib is not a substrate of CYP enzymes in vitro.
Effect of Trametinib on CYP Substrates: Based on in vitro studies, trametinib is an inhibitor of CYP2C8, but is not an inhibitor of CYP1A2, CYP2A6, CYP2B6, CYP2C9, CYP2C19, CYP2D6, or CYP3A4 at a clinically relevant systemic concentration of 0.04 µM. Trametinib is an inducer of CYP3A in vitro. Based on cross-study comparisons, oral administration of MEKINIST 2 mg once daily with a sensitive CYP3A4 substrate had no clinically important effect on the AUC and Cmax of the sensitive CYP3A4 substrate.
Effect of Transporters on Trametinib: Trametinib is a substrate of P-glycoprotein (P-gp) and bile salt extrusion pump (BSEP). Inhibition of P-gp is unlikely to result in a clinically important increase in trametinib concentrations as trametinib exhibits high passive permeability and bioavailability. Trametinib is not a substrate of breast cancer resistance protein (BCRP), organic anion transporting polypeptide (OATP1B1, OATP1B3, OATP2B1), organic cation transporter 1 (OCT1), multidrug resistance protein 2 (MRP2), or multidrug and toxin extrusion 1 (MATE1) in vitro.
Effect of Trametinib on Transporters: Based on in vitro studies, trametinib is not an inhibitor of P-gp, BCRP, OATP1B1, OATP1B3, organic anion transporter (OAT1, OAT3), OCT2, BSEP, MRP2, or MATE1 at a clinically relevant systemic concentration of 0.04 µM.
Effect of Dabrafenib on Trametinib: Coadministration of trametinib 2 mg daily with dabrafenib 150 mg twice daily resulted in no change in AUC of trametinib as compared with administration of trametinib.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies with trametinib have not been conducted. Trametinib was not genotoxic in studies evaluating reverse mutations in bacteria, chromosomal aberrations in mammalian cells, and micronuclei in the bone marrow of rats.
Trametinib may impair fertility in humans. In female rats given trametinib for up to 13 weeks, increased follicular cysts and decreased corpora lutea were observed at doses ≥0.016 mg/kg/day (approximately 0.3 times the human exposure at the recommended dose based on AUC). In rat and dog toxicity studies up to 13 weeks in duration, there were no treatment effects observed on male reproductive tissues. [see Use in Specific Populations (8.3)].
14 CLINICAL STUDIES
14.1 BRAF V600E or V600K Mutation-Positive Unresectable or Metastatic Melanoma
Mekinist as a Single Agent
The safety and efficacy of MEKINIST were evaluated in an international, multicenter, randomized (2:1), open-label, active-controlled trial (Trial 1) in 322 patients with BRAF V600E or V600K mutation-positive, unresectable or metastatic melanoma.
In Trial 1, patients were not permitted to have more than one prior chemotherapy regimen for advanced or metastatic disease; prior treatment with a BRAF inhibitor or MEK inhibitor was not permitted. The primary efficacy outcome measure was progression-free survival (PFS). Patients were randomized to receive MEKINIST 2 mg orally once daily (N = 214) or chemotherapy (N = 108) consisting of either dacarbazine 1,000 mg/m2 intravenously every 3 weeks or paclitaxel 175 mg/m2 intravenously every 3 weeks. Treatment continued until disease progression or unacceptable toxicity. Randomization was stratified according to prior use of chemotherapy for advanced or metastatic disease (yes versus no) and lactate dehydrogenase level (normal versus greater than upper limit of normal). Tumor tissue was evaluated for BRAF mutations at a central testing site using a clinical trial assay. Tumor samples from 289 patients (196 patients treated with MEKINIST and 93 chemotherapy-treated patients) were also tested retrospectively using an FDA-approved companion diagnostic test, THxID™-BRAF assay.
The median age for randomized patients was 54 years, 54% were male, greater than 99% were White, and all patients had baseline ECOG performance status of 0 or 1. Most patients had metastatic disease (94%), were Stage M1c (64%), had elevated LDH (36%), had no history of brain metastasis (97%), and received no prior chemotherapy for advanced or metastatic disease (66%). The distribution of BRAF V600 mutations was BRAF V600E (87%), V600K (12%), or both (less than 1%). The median durations of follow-up prior to initiation of alternative treatment were 4.9 months for patients treated with MEKINIST and 3.1 months for patients treated with chemotherapy. Fifty-one (47%) patients crossed over from the chemotherapy arm at the time of disease progression to receive MEKINIST.
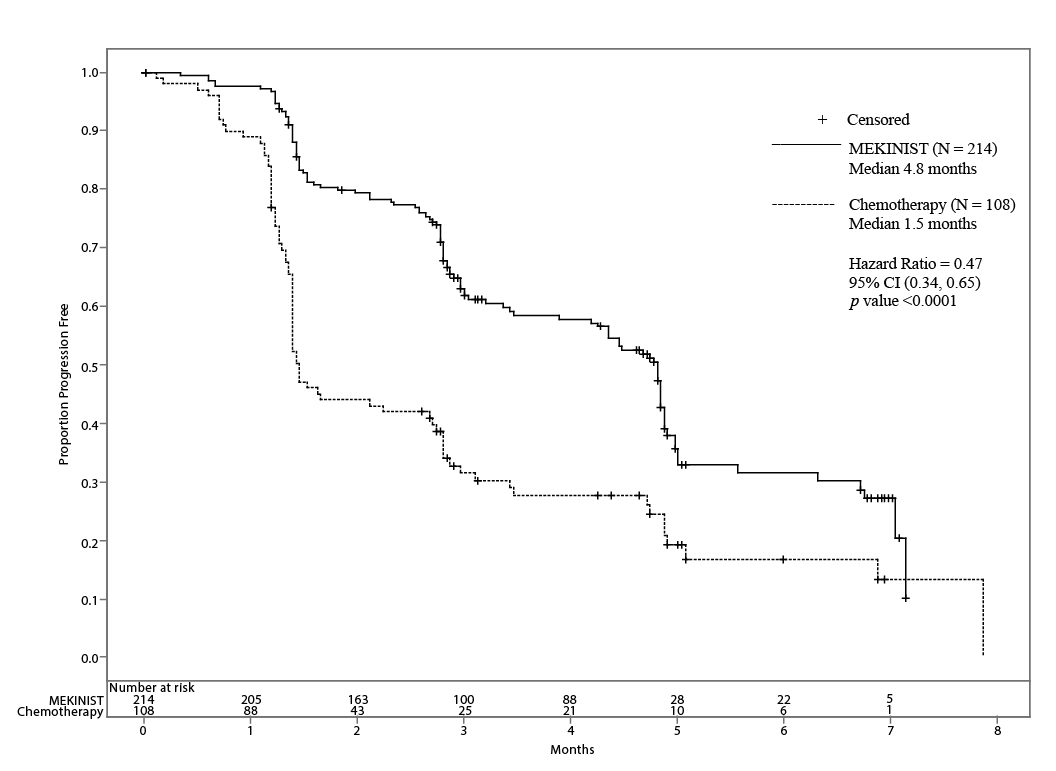
Trial 1 demonstrated a statistically significant increase in progression-free survival in the patients treated with MEKINIST. Table 7 and Figure 1 summarize the PFS results.
Table 7. Investigator-Assessed Progression-Free Survival and Confirmed Objective Response Results in Trial 1
|
Investigator-Assessed Endpoints |
MEKINIST N = 214 |
Chemotherapy N = 108 |
|
Progression-Free Survival | ||
|
117 (55%) |
77 (71%) |
|
107 (50%) |
70 (65%) |
|
10 (5%) |
7 (6%) |
|
4.8 (4.3, 4.9) |
1.5 (1.4, 2.7) |
|
0.47 (0.34, 0.65) |
|
|
P<0.0001 |
|
|
Confirmed Tumor Responses | ||
|
22% |
8% |
|
(17, 28) |
(4, 15) |
|
4 (2%) |
0 |
|
43 (20%) |
9 (8%) |
| ||
|
5.5 (4.1, 5.9) |
NR (3.5, NR) |
CI = Confidence interval; HR = Hazard ratio; CR = Complete response; PR = Partial response; NR = Not reached.
aPike estimator.
Figure 1. Kaplan-Meier Curves of Investigator-Assessed Progression-Free Survival (ITT Population) in Trial 1
In supportive analyses based on independent radiologic review committee (IRRC) assessment, the PFS results were consistent with those of the primary efficacy analysis.
Mekinist with Dabrafenib
The safety and efficacy of MEKINIST administered with dabrafenib were evaluated in an international, randomized, double-blind, active-controlled trial (Trial 2). Trial 2 compared dabrafenib plus MEKINIST to dabrafenib plus placebo as first-line treatment for patients with unresectable (Stage IIIc) or metastatic (Stage IV) BRAF V600E or V600K mutation-positive cutaneous melanoma. Patients were randomized (1:1) to receive MEKINIST 2 mg once daily plus dabrafenib 150 mg twice daily or dabrafenib 150 mg twice daily plus matching placebo. Randomization was stratified by lactate dehydrogenase (LDH) level (greater than the upper limit of normal (ULN) vs. ≤ ULN) and BRAF mutation subtype (V600E vs. V600K). The major efficacy outcome was investigator-assessed progression-free survival (PFS) per RECIST v1.1 with additional efficacy outcome measures of overall survival (OS) and confirmed overall response rate (ORR).
In Trial 2, 423 patients were randomized to MEKINIST plus dabrafenib (n = 211) or dabrafenib plus placebo (n = 212). The median age was 56 years (range: 22 to 89 years), 53% were male, >99% were White, 72% had ECOG performance status of 0, 4% had Stage IIIc, 66% had M1c disease, 65% had a normal LDH, and 2 patients had a history of brain metastases. All patients had tumor containing BRAF V600E or V600K mutations as determined by centralized testing with the FDA-approved companion diagnostic test; 85% had BRAF V600E mutation-positive melanoma and 15% had BRAF V600K mutation-positive melanoma.
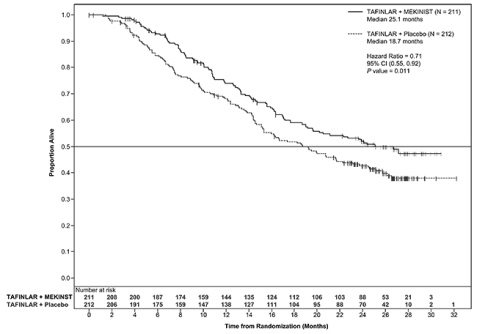
Trial 2 demonstrated statistically significant improvements in PFS and OS (see Table 8 and Figure 2).
Table 8. Efficacy Results in Trial 2
|
Endpoint† |
MEKINIST plus dabrafenib N=211 |
Dabrafenib plus Placebo N=212 |
|
Progression-Free Survival (PFS)a |
||
|
Number of events (%) |
102 (48%) |
109 (51%) |
|
9.3 (7.7, 11.1) |
8.8 (5.9, 10.9) |
|
0.75 (0.57, 0.99) |
|
|
0.035 |
|
|
Overall Survival |
||
|
99 (47% ) |
123 (58%) |
|
25.1 (19.2, NR) |
18.7 (15.2, 23.1) |
|
0.71 (0.55, 0.92) |
|
|
0.01 |
|
|
Overall Response Rate (ORR)b |
||
|
66 (60, 73) |
51 (44, 58) |
|
<0.001 |
|
|
10 |
8 |
|
56 |
42 |
|
9.2 (7.4, NR) |
10.2 (7.5, NR) |
- † CI = Confidence interval; HR = Hazard ratio; CR = Complete response; PR = Partial response; NR = Not reached.
- a PFS and ORR were assessed by investigator.
- bBased on stratified log-rank test
Figure 2. Kaplan Meier Curves of Overall Survival in Trial 2
14.2 Lack of Clinical Activity in Metastatic Melanoma Following BRAF-Inhibitor Therapy
The clinical activity of MEKINIST as a single agent was evaluated in a single-arm, multicenter, international trial in 40 patients with BRAF V600E or V600K mutation-positive, unresectable or metastatic melanoma who had received prior treatment with a BRAF inhibitor. All patients received MEKINIST at a dose of 2 mg orally once daily until disease progression or unacceptable toxicity.
The median age was 58 years, 63% were male, all were White, 98% had baseline ECOG PS of 0 or 1, and the distribution of BRAF V600 mutations was V600E (83%), V600K (10%), and the remaining patients had multiple V600 mutations (5%), or unknown mutational status (2%). No patient achieved a confirmed partial or complete response as determined by the clinical investigators.
16 HOW SUPPLIED/STORAGE AND HANDLING
0.5 mg tablets: Yellow, modified oval, biconvex, film-coated tablets with ‘GS’ debossed on one face and ‘TFC’ on the opposing face and are available in bottles of 30 (NDC 0173-0849-13).
2 mg tablets: Pink, round, biconvex, film-coated tablets with ‘GS’ debossed on one face and ‘HMJ’ on the opposing face and are available in bottles of 30 (NDC 0173-0848-13).
Store refrigerated at 2° to 8°C (36° to 46°F). Do not freeze. Dispense in original bottle. Do not remove desiccant. Protect from moisture and light. Do not place medication in pill boxes.
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Inform patients of the following:
Confirmation of BRAF V600E or V600K mutation
Evidence of BRAF V600E or V600K mutation within the tumor specimen is necessary to identify patients for whom treatment with MEKINIST is indicated [see Dosage and Administration (2.1)].
New cutaneous and non-cutaneous malignancies
MEKINIST administered with dabrafenib can result in the development of new primary cutaneous and non-cutaneous malignancies. Advise patients to contact their doctor immediately for any new lesions, changes to existing lesions on their skin, or other signs and symptoms of malignancies [see Warnings and Precautions (5.1)].
Hemorrhage
MEKINIST administered with dabrafenib increases the risk of intracranial and gastrointestinal hemorrhage. Advise patients to contact their healthcare provider to seek immediate medical attention for signs or symptoms of unusual bleeding or hemorrhage [see Warnings and Precautions (5.2)].
Venous thrombosis
MEKINIST administered with dabrafenib increases the risks of pulmonary embolism and deep venous thrombosis. Advise patients to seek immediate medical attention for sudden onset of difficulty breathing, leg pain, or swelling [see Warnings and Precautions (5.3)].
Cardiomyopathy
MEKINIST can cause cardiomyopathy. Advise patients to immediately report any signs or symptoms of heart failure to their healthcare provider [see Warnings and Precautions (5.4)].
Retinal Pigment Epithelial Detachment
MEKINIST can cause severe visual disturbances that can lead to blindness. Advise patients to contact their healthcare provider if they experience any changes in their vision [see Warnings and Precautions (5.5)].
Interstitial lung disease
MEKINIST can cause interstitial lung disease (or pneumonitis). Advise patients to contact their healthcare provider as soon as possible if they experience signs such as cough or dyspnea [see Warnings and Precautions (5.6)].
Serious febrile reactions
MEKINIST administered with dabrafenib can cause serious febrile reactions. Instruct patients to contact their healthcare provider if they develop fever while taking MEKINIST with dabrafenib [see Warnings and Precautions (5.7)].
Serious skin toxicities
MEKINIST can cause serious skin toxicities which may require hospitalization. Advise patients to contact their healthcare provider for progressive or intolerable rash [see Warnings and Precautions (5.8)].
Hypertension
MEKINIST can cause hypertension. Advise patients that they need to undergo blood pressure monitoring and to contact their healthcare provider if they develop symptoms of hypertension such as severe headache, blurry vision, or dizziness.
Diarrhea
MEKINIST often causes diarrhea which may be severe in some cases. Inform patients of the need to contact their healthcare provider if severe diarrhea occurs during treatment.
Embryo-fetal Toxicity
MEKINIST can cause fetal harm if taken during pregnancy. Advise a pregnant woman of the potential risk to a fetus [see Warnings and Precautions (5.10), Use in Specific Populations (8.1, 8.3)].
Females and males of reproductive potential
Instruct females of reproductive potential to use highly effective contraception during treatment with MEKINIST and for 4 months after the last dose. Advise patients to contact their healthcare provider if they become pregnant, or if pregnancy is suspected, while taking MEKINIST [see Warnings and Precautions (5.10), Use in Specific Populations (8.1, 8.3)].
Lactation
Advise women not to breastfeed during treatment with MEKINIST and for 4 months after the last dose [see Use in Specific Populations (8.2)].
Infertility
Advise males and females of reproductive potential of the potential risk for impaired fertility [see Use in Specific Populations (8.3)].
Instructions for taking MEKINIST
MEKINIST should be taken at least 1 hour before or at least 2 hours after a meal [seeDosage and Administration (2.2)].
MEKINIST is a registered trademark of the GSK group of companies.
THxID BRAF™ assay is a trademark of bioMérieux.
GlaxoSmithKline
Research Triangle Park, NC 27709
©2015, the GSK group of companies. All rights reserved.
MKN:3PI
MEKINIST® (MEK-in-ist) (trametinib) tablets |
|
If your healthcare provider prescribes MEKINIST for you to be taken with dabrafenib, also read the Medication Guide that comes with dabrafenib. |
|
What is the most important information I should know about MEKINIST? MEKINIST, when used with dabrafenib, may cause:
Talk to your healthcare provider about your risk for these cancers. Check your skin and tell your healthcare provider right away about any skin changes including a:
Your healthcare provider should check your skin before treatment with MEKINIST and dabrafenib, every two months during treatment with MEKINIST and dabrafenib and for up to 6 months after you stop taking MEKINIST and dabrafenib to look for any new skin cancers. Your healthcare provider should also check for cancers that may not occur on the skin. Tell your healthcare provider about any new symptoms that develop during treatment with MEKINIST with dabrafenib. See "What are the possible side effects of MEKINIST?" for more information about side effects. |
|
What is MEKINIST? MEKINIST is a prescription medicine used by itself or with a medicine called dabrafenib, to treat people with a type of skin cancer called melanoma:
Your healthcare provider will perform a test to make sure that MEKINIST is right for you. MEKINIST should not be used to treat people who already have received a BRAF inhibitor for treatment of their melanoma. It is not known if MEKINIST alone or MEKINIST with dabrafenib is safe and effective in children. |
|
What should I tell my healthcare provider before taking MEKINIST? Before you take MEKINIST, tell your healthcare provider if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine. |
|
How should I take MEKINIST?
|
|
What are the possible side effects of MEKINIST? MEKINIST may cause serious side effects, including:
The most common side effects of MEKINIST when taken alone include:
Common side effects of MEKINIST when taken with dabrafenib include: • nausea • vomiting • chills • high blood pressure (hypertension) • diarrhea • swelling of the face, arms, or legs MEKINIST can cause new or worsening high blood pressure (hypertension). Your healthcare provider should check your blood pressure during treatment with MEKINIST. Call your healthcare provider right away if you develop high blood pressure, your blood pressure worsens, or you have severe headache, lightheadedness, or dizziness. MEKINIST may cause fertility problems in females. This could affect your ability to become pregnant. Talk to your healthcare provider if this is a concern for you. Tell your healthcare provider if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of MEKINIST. For more information, ask your healthcare provider or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|
How should I store MEKINIST?
Keep MEKINIST and all medicine out of the reach of children. |
|
General information about the safe and effective use of MEKINIST. Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use MEKINIST for a condition for which it was not prescribed. Do not give MEKINIST to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information about MEKINIST that is written for health professionals. For more information, go to www.MEKINIST.com or call 1-888-825-5249. |
|
What are the ingredients in MEKINIST? Active ingredient: trametinib Inactive ingredients: Tablet Core: colloidal silicon dioxide, croscarmellose sodium, hypromellose, magnesium stearate (vegetable source), mannitol, microcrystalline cellulose, sodium lauryl sulfate. Tablet Coating: hypromellose, iron oxide red (2 mg tablets), iron oxide yellow (0.5 mg tablets), polyethylene glycol, polysorbate 80 (2 mg tablets), titanium dioxide. GlaxoSmithKline Research Triangle Park, NC 27709 Revised: November 2015 MEKINIST is a registered trademark of the GSK group of companies. ©2015, the GSK group of companies. All rights reserved. MKN:3PL |
PRINCIPAL DISPLAY PANEL
NDC 0173-0849-13
Mekinist®
(trametinib) Tablets
0.5 mg*
Rx only
30 Tablets
*Each tablet contains 0.5635 mg trametinib dimethyl sulfoxide equivalent to 0.5 mg of trametinib.
Dosage: See accompanying prescribing information.
Store refrigerated at 2° and 8°C (36° to 46°F).
Do not freeze.
Do not use if printed safety seal under cap is broken or missing.
Dispense in original bottle. Do not remove desiccant. Protect from moisture and light. Do not place medication in pill boxes.
GlaxoSmithKline
RTP, NC 27709
Made in Singapore
- 10000000124168 Rev. 2/14
PRINCIPAL DISPLAY PANEL
NDC 0173-0848-13
Mekinist®
(trametinib) Tablets
2 mg*
Rx only
30 Tablets
*Each tablet contains 2.254 mg trametinib dimethyl sulfoxide equivalent to 2 mg of trametinib.
Dosage: See accompanying prescribing information.
Store refrigerated at 2° and 8°C (36° to 46°F).
Do not freeze.
Do not use if printed safety seal under cap is broken or missing.
Dispense in original bottle. Do not remove desiccant. Protect from moisture and light. Do not place medication in pill boxes.
GlaxoSmithKline
RTP, NC 27709
Made in Singapore
- 10000000124203 Rev. 3/14
| MEKINIST
trametinib tablet, film coated |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| MEKINIST
trametinib tablet, film coated |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - GlaxoSmithKline LLC (167380711) |