Label: GONAL-F- follitropin alfa kit
- NDC Code(s): 44087-9030-1, 44087-9070-1
- Packager: EMD Serono, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated November 29, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use GONAL-F® safely and effectively. See full prescribing information for Gonal-F®
GONAL-F® (follitropin alfa) for injection, for subcutaneous use
Initial U.S. Approval: 1997INDICATIONS AND USAGE
GONAL-F is a gonadotropin indicated for:
- Women:
- Men:
- Induction of spermatogenesis in infertile men with primary and secondary hypogonadotropic hypogonadism for whom the cause of infertility is not due to primary testicular failure. (1.3)
DOSAGE AND ADMINISTRATION
Induction of Ovulation (2.3)
- Initial starting dose of the first cycle - 75 International Units of GONAL-F per day for 14 days, administered subcutaneously
- Individualize doses after 14 days
- Do not administer doses greater than 300 International Units per day
Development of Multiple Follicles in Assisted Reproductive Technology (ART) (2.3)
- Initial starting dose of the first cycle - 150 International Units per day, administered subcutaneously
- Dosage adjustments after 3 to 5 days and by 75 to 150 International Units at each adjustment
- Do not administer doses greater than 450 International Units per day
Males with Hypogonadotropic Hypogonadism and Azoospermia (2.4)
- Use in conjunction with hCG.
- Prior to concomitant therapy with GONAL-F and hCG, pretreat with 1,000 to 2,250 USP units of hCG alone two to three times per week to achieve normal serum testosterone levels, which may take 3 to 6 months.
- After normalization of serum testosterone, administer150 International Units of GONAL-F subcutaneously three times a week and 1,000 USP units of hCG (or the dose required to maintain serum testosterone levels within the normal range) three times a week.
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
GONAL-F is contraindicated in women and men who exhibit (4):
- Prior hypersensitivity to recombinant FSH products or one of their excipients
- High levels of FSH indicating primary gonadal failure
- Uncontrolled non-gonadal endocrinopathies
- Sex hormone dependent tumors of the reproductive tract and accessory organs
- Tumors of pituitary gland or hypothalamus
GONAL-F is also contraindicated in women who exhibit (4):
- Abnormal uterine bleeding of undetermined origin
- Ovarian cyst or enlargement of undetermined origin
WARNINGS AND PRECAUTIONS
- Hypersensitivity Reactions and Anaphylaxis: If occurs, initiate appropriate therapy including supportive measures, and discontinue Gonal-F (5.1)
- Ovarian Hyperstimulation Syndrome: If serious, stop gonadotropins, including hCG, and determine if the woman needs to be hospitalized. Treatment is primarily symptomatic and consists of bed rest, fluid and electrolyte management, and analgesics (5.2)
- Pulmonary and Vascular Complications: In women with recognized risk factors, the benefits of induction of ovulation and ART need to be weighed against the risks. During or after use of GONAL-F, monitor for venous or arterial thromboembolic events (5.3 )
- Ovarian Torsion: Early diagnosis and immediate detorsion limit damage to the ovary due to reduced blood supply (5.4)
- Abnormal Ovarian Enlargement: If the ovaries are abnormally enlarged on the last day of GONAL-F therapy, inform women not to administer hCG and to avoid intercourse (5.5)
- Multi-fetal Gestation and Births: The rate of multiple births is dependent on the number of embryos transferred. Advise the woman and her partner of the potential risk of multi-fetal gestation and birth before beginning therapy with GONAL-F (5.6)
- Embryofetal Toxicity: Inform women that the incidence of congenital malformations (birth defects) after some Assisted Reproductive Technology [(ART) specifically in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI)] may be slightly higher than after spontaneous conception. There is no evidence that the use of gonadotropins during IVF or ICSI is associated with an increased risk of congenital malformations (5.7)
- Ectopic Pregnancy: Advise women who become pregnant following ART and have: abdominal/pelvic pain (particularly on one side); shoulder, neck or rectal pain; and nausea and vomiting to seek immediate medical attention. Confirm the presence of an intrauterine pregnancy early by β-hCG testing and transvaginal ultrasound (5.8)
- Spontaneous Abortion: The risk of spontaneous abortion (miscarriage) is increased with gonadotropin products, however, causality has not been established (5.9)
- Ovarian Neoplasm: Both benign and malignant ovarian neoplasms are reported in women who have had multiple drug therapy for controlled ovarian stimulation, however, causality has not been established (5.10)
ADVERSE REACTIONS
- The most common adverse reactions (≥5%) in ovulation induction include: ovarian cyst,headache, abdominal pain, OHSS, nausea, flatulence, pain and intermenstrual bleeding. (6.1)
- The most common adverse reactions (≥5%) in development of multiple follliclesg in ART include: headache, nausea, pelvic pain and abdominal pain. (6.1)
- The most common adverse reactions (>5%) in hypogonadotropic hypogonadal men participating in induction of spermatogenesis include: acne, injection site pain, fatigue, gynecomastia and seborrhea (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact EMD Serono at 1-800-283-8088, Ext 5563 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 11/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Induction of ovulation and pregnancy in oligo-anovulatory infertile women for whom the cause of infertility is functional and not due to primary ovarian failure.
1.2 Development of multiple follicles in ovulatory infertile women as part of an assisted reproductive technology (ART) cycle.
1.3 Induction of spermatogenesis in infertile men with primary and secondary hypogonadotropic hypogonadism for whom the cause of infertility is not due to primary testicular failure.
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Information
2.2 Preparation of GONAL-F and Selection of Injection Site
2.3 Dosing for Ovulation Induction
2.4 Dosing for Multiple Follicle Development as part of an Assisted Reproductive Technology (ART) Cycle
2.5 Dosing for Induction of Spermatogenesis in Males with Azoospermia and Primary or Secondary Hypogonadotropic Hypogonadism:
2.6 Missed Dose
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions and Anaphylaxis
5.2 Ovarian Hyperstimulation Syndrome (OHSS)
5.3 Pulmonary and Vascular Complications
5.4 Ovarian Torsion
5.5 Abnormal Ovarian Enlargement
5.6 Multi-fetal Gestation and Birth
5.7 Embryofetal toxicity
5.8 Ectopic Pregnancy
5.9 Spontaneous Abortion
5.10 Ovarian Neoplasms
5.11 Laboratory Tests
6 ADVERSE REACTIONS
6.1 Clinical Study Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Induction of Ovulation
14.2 Development of Multiple Follicles as part of an Assisted Reproductive Technology (ART) Cycle:
14.3 Induction of Spermatogenesis in Males
16 How Supplied/Storage and Handling
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
Gonal-F is indicated for:
1.1 Induction of ovulation and pregnancy in oligo-anovulatory infertile women for whom the cause of infertility is functional and not due to primary ovarian failure.
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Information
Only physicians who are experienced in infertility treatment, should treat women with GONAL-F. GONAL-F is a gonadotropins product capable of causing in women, Ovarian Hyperstimulation Syndrome (OHSS) with or without pulmonary or vascular complications [see Warnings and Precautions (5.2, 5.3)] and multiple births [see Warnings and Precautions (5.6)]. Gonadotropin therapy requires the availability of appropriate monitoring facilities [see Warnings and Precautions (5.11)]. Use the lowest effective dose of GONAL-F.
Give careful attention to the diagnosis of infertility and the selection of candidates for GONAL-F therapy [see Dosage and Administration (2.3, 2.4)].
2.2 Preparation of GONAL-F and Selection of Injection Site
- Store lyophilized multiple-dose vials refrigerated or at room temperature (2°-25°C /36°-77°F) and protected from light.
- Prior to administration, parenteral drug products should be inspected visually for particulate matter and discoloration, whenever solution and container permit.
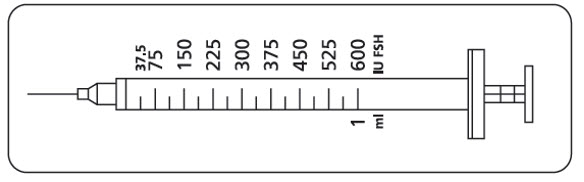
- Instruct women and men to use the accompanying syringes, calibrated in International Units FSH for administration. The 27-gauge injection syringe (see figure below) has unit dose markings from 37.5 International Units to 600 International Units FSH for use with GONAL-F Multi-Dose. Instruct women and men to take a specific dose of GONAL-F Multi-Dose. Show women and men how to locate the syringe marking that corresponds to the prescribed dose.
- Each GONAL‑F Multi‑Dose Vial delivers 450 International Units or 1050 International Units of follitropin alfa, respectively
-
Multi-Dose 450 International Units Vial:
- Dissolve the contents of one Multi-Dose vial (450 International Units) with 1 mL Bacteriostatic Water for Injection (0.9% benzyl alcohol), USP. Resulting concentration will be 600 International Units/mL. Following reconstitution as directed, product will deliver the equivalent of six 75 International Units doses.
-
Multi-Dose 1050 International Units Vial:
- Dissolve the contents of one Multi-Dose vial (1050 International Units) with 2 mL Bacteriostatic Water for Injection (0.9% benzyl alcohol), USP. Resulting concentration will be 600 International Units/mL. Following reconstitution as directed, product will deliver the equivalent of fourteen 75 International Units doses.
-
Multi-Dose 450 International Units Vial:
- Discard unused reconstituted solution after 28 days.
- Administer GONAL-F subcutaneously in the abdomen, upper arm, or upper leg as described in Patient Information and Instructions for Use.
2.3 Dosing for Ovulation Induction
Prior to initiation of treatment with GONAL-F:
- Perform a complete gynecologic and endocrinologic evaluation
- Exclude primary ovarian failure
- Exclude the possibility of pregnancy
- Demonstrate tubal patency
- Evaluate the fertility status of the male partner
The dosing scheme is stepwise and is individualized for each woman [see Clinical Studies (14.1)].
- Administer a starting dose of 75 International Units of GONAL-F subcutaneously daily for 14 days in the first cycle of use.
- In subsequent cycles of treatment, determine the starting dose (and dosage adjustments) of GONAL-F based on the woman's history of the ovarian response to GONAL-F.
- If indicated by the ovarian response after the initial 14 days, make an incremental adjustment in dose of up to 37.5 International Units.
- If indicated by the ovarian response, make additional incremental adjustments in the dose, up to 37.5 International Units, every 7 days.
- Continue treatment until follicular growth and/or serum estradiol levels indicate an adequate ovarian response.
- Consider the following when planning the woman's individualized dose:
- Use the lowest dose of Gonal-F consistent with the expectation of good results.
- Use appropriate GONAL-F dose adjustment(s) to prevent multiple follicular growth and cycle cancellation.
- The maximum, individualized, daily dose of GONAL-F is 300 International Units per day.
- In general, do not exceed 35 days of treatment, unless an estradiol rise indicates imminent follicular development.
- When pre-ovulatory conditions are reached, administer human chorionic gonadotropin (hCG) to induce final oocyte maturation and ovulation. Human chorionic gonadotropin, hCG, (5,000 USP units) should be given 1 day after the last dose of GONAL-F.
- Encourage the woman and her partner to have intercourse daily, beginning on the day prior to the administration of hCG and until ovulation becomes apparent.
- Withhold hCG in cases where the ovarian monitoring suggests an increased risk of ovarian hyperstimulation syndrome (OHSS) on the last day of GONAL-F therapy (for example estradiol greater than 2,000 pg per mL) [see Warnings and Precautions (5.2, 5.3, 5.5, 5.11)].
- Discourage intercourse when the risk for OHSS is increased [see Warnings and Precautions (5.2, 5.5)].
- Schedule a follow-up visit in the luteal phase.
- Individualize the initial dose administered in subsequent cycles based on the woman's response in the preceding cycle.
- As in the initial cycle, do not administer doses larger than 300 International Units of FSH per day. Administer 5,000 USP units of hCG 1 day after the last dose of GONAL-F to complete follicular development and induce ovulation.
- Follow the above recommendations to minimize the chance of development of OHSS.
2.4 Dosing for Multiple Follicle Development as part of an Assisted Reproductive Technology (ART) Cycle
Prior to initiation of treatment with GONAL-F:
- Perform a complete gynecologic and endocrinologic evaluation, and diagnose the cause of infertility
- Exclude the possibility of pregnancy
- Evaluate the fertility status of the male partner
The dosing scheme follows a stepwise approach and is individualized for each woman.
- Beginning on cycle day 2 or 3, administer subcutaneously a starting dose of 150 International Units of GONAL-F daily until sufficient follicular development, as determined by ultrasound in combination with measurement of serum estradiol levels, is attained. In most cases, therapy should not exceed ten days.
- In women whose endogenous gonadotropin levels are suppressed, initiate GONAL-F administration at a dose of 225 International Units per day.
- Adjust the dose after 5 days based on the woman's ovarian response, as determined by ultrasound evaluation of follicular growth and serum estradiol levels.
- Do not make additional dosage adjustments more frequently than every 3-5 days or by more than 75-150 International Units at each adjustment.
- Continue treatment until adequate follicular development is evident, and then administer hCG (5,000 to 10,000 USP units) to induce final follicular maturation in preparation for oocyte retrieval.
- Withhold hCG administration in cases where the ovarian monitoring suggests an increased risk of OHSS on the last day of GONAL-F therapy [see Warnings and Precautions (5.2, 5.3, 5.4, 5.11)].
- Do not use doses greater than 450 International Units per day.
2.5 Dosing for Induction of Spermatogenesis in Males with Azoospermia and Primary or Secondary Hypogonadotropic Hypogonadism:
Prior to initiation of treatment with GONAL-F:
- Confirm azoospermia
- Perform a thorough medical and endocrinologic evaluation to exclude other treatable etiologies of azoospermia
- Confirm hypogonadotropic hypogonadism
- Exclude primary testicular failure
- Normalize serum testosterone levels
The dosing scheme follows a stepwise approach and is individualized for each man.
- GONAL-F must be given in conjunction with hCG.
- Prior to concomitant therapy with GONAL-F and hCG, pretreatment with hCG alone (1,000 to 2,250 USP units two to three times per week) is required to normalize serum testosterone levels.
- Treatment with hCG alone should continue until serum testosterone levels reach the normal range, which may take 3 to 6 months. The dose of hCG may also need to be increased during this time to achieve normal serum testosterone levels.
- After serum testosterone levels have normalized, administer GONAL-F 150 International Units subcutaneously three times a week and hCG 1,000 USP units (or the dose required to maintain serum testosterone levels within the normal range) three times a week. The lowest dose of GONAl-F which induces spermatogenesis should be utilized.
- If azoospermia persists, increase the dose of GONAL-F up to a maximum dose of 300 International Units three times per week. Administer GONAL-F for up to 18 months to achieve adequate spermatogenesis.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
GONAL-F is contraindicated in women and men who exhibit:
- Prior hypersensitivity to recombinant FSH products or one of their excipients. Reactions have included anaphylaxis [see Warning and Precautions (5.1)]
- High levels of FSH indicating primary gonadal failure
- The presence of uncontrolled non-gonadal endocrinopathies (for example, thyroid, adrenal, or pituitary disorders)
- Sex hormone dependent tumors of the reproductive tract and accessory organs
- Tumors of pituitary gland or hypothalamus
GONAL-F is also contraindicated in women who exhibit:
- Abnormal uterine bleeding of undetermined origin
- Ovarian cyst or enlargement of undetermined origin
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions and Anaphylaxis
In the postmarketing experience, serious systemic hypersensitivity reactions, including anaphylaxis, have been reported with use of GONAL-F and GONAL-F RFF. Symptoms have included dyspnea, facial edema, pruritis, and urticaria. If an anaphylactic or other serious allergic reaction occurs, initiate appropriate therapy including supportive measures if cardiovascular instability and/or respiratory compromise occur, and discontinue further use.
5.2 Ovarian Hyperstimulation Syndrome (OHSS)
Ovarian Hyperstimulation Syndrome (OHSS) is a medical entity distinct from uncomplicated ovarian enlargement and may progress rapidly to become a serious medical event. OHSS is characterized by a dramatic increase in vascular permeability, which can result in a rapid accumulation of fluid in the peritoneal cavity, thorax, and potentially, the pericardium. The early warning signs of development of OHSS are severe pelvic pain, nausea, vomiting, and weight gain. Abdominal pain, abdominal distension, gastrointestinal symptoms including nausea, vomiting and diarrhea, severe ovarian enlargement [see Warnings and Precautions (5.6)], weight gain, dyspnea, and oliguria have been reported with OHSS. Clinical evaluation may reveal hypovolemia, hemoconcentration, electrolyte imbalances, ascites, hemoperitoneum, pleural effusions, hydrothorax, acute pulmonary distress, and thromboembolic reactions [see Warnings and Precautions (5.4)]. Transient liver function test abnormalities suggestive of hepatic dysfunction with or without morphologic changes on liver biopsy, have been reported in association with OHSS.
OHSS occurs after gonadotropin treatment has been discontinued and it can develop rapidly, reaching its maximum about seven to ten days following treatment. Usually, OHSS resolves spontaneously with the onset of menses. If there is evidence that OHSS may be developing prior to hCG administration [see Dosage and Administration (2.2, 2.3)], withhold hCG. Cases of OHSS are more common, more severe, and more protracted if pregnancy occurs; therefore, assess women for the development of OHSS for at least two weeks after hCG administration.
If serious OHSS occurs, stop gonadotropins, including GONAL-F and hCG, and consider whether the woman needs to be hospitalized. Treatment is primarily symptomatic and overall consists of bed rest, fluid and electrolyte management, and analgesics (if needed). Because the use of diuretics can accentuate the diminished intravascular volume, avoid diuretics except in the late phase of resolution as described below. The management of OHSS is divided into three phases as follows:
-
Acute Phase:
Management is directed at preventing hemoconcentration due to loss of intravascular volume to the third space and minimizing the risk of thromboembolic phenomena and kidney damage. Thoroughly assess daily or more often, based on the clinical need, fluid intake and output, weight, hematocrit, serum and urinary electrolytes, urine specific gravity, BUN and creatinine, total proteins with albumin: globulin ratio, coagulation studies, electrocardiogram to monitor for hyperkalemia, and abdominal girth. Treatment, consisting of limited intravenous fluids, electrolytes, human serum albumin, is intended to normalize electrolytes while maintaining an acceptable but somewhat reduced intravascular volume. Full correction of the intravascular volume deficit may lead to an unacceptable increase in the amount of third space fluid accumulation. -
Chronic Phase:
After the acute phase is successfully managed as above, excessive fluid accumulation in the third space should be limited by instituting severe potassium, sodium, and fluid restriction. -
Resolution Phase:
As third space fluid returns to the intravascular compartment, a fall in hematocrit and increasing urinary output are observed in the absence of any increase in intake. Peripheral and/or pulmonary edema may result if the kidneys are unable to excrete third space fluid as rapidly as it is mobilized. Diuretics may be indicated during the resolution phase, if necessary, to combat pulmonary edema.
Do not remove ascitic, pleural, and pericardial fluid, unless there is the necessity to relieve symptoms such as pulmonary distress or cardiac tamponade.
OHSS increases the risk of injury to the ovary. Avoid pelvic examination or intercourse, as these may cause rupture of an ovarian cyst, which may result in hemoperitoneum.
If bleeding occurs and requires surgical intervention, control the bleeding and retain as much ovarian tissue as possible. A physician experienced in the management of this syndrome, or who is experienced in the management of fluid and electrolyte imbalances should be consulted.
5.3 Pulmonary and Vascular Complications
Serious pulmonary conditions (for example, atelectasis, acute respiratory distress syndrome and exacerbation of asthma) have been reported in women treated with gonadotropins, including GONAL-F. In addition, thromboembolic events both in association with, and separate from OHSS have been reported in women treated with gonadotropins, including GONAL-F. Intravascular thrombosis and embolism, which may originate in venous or arterial vessels, can result in reduced blood flow to critical organs or the extremities. Women with generally recognized risk factors for thrombosis, such as personal or family history, severe obesity, or thrombophilia, may have an increased risk of venous or arterial thromboembolic events, during or following treatment with gonadotropins. Sequelae of such reactions have included venous thrombophlebitis, pulmonary embolism, pulmonary infarction, cerebral vascular occlusion (stroke), and arterial occlusion resulting in loss of limb and rarely in myocardial infarctions. In rare cases, pulmonary complications and/or thromboembolic reactions have resulted in death. In women with recognized risk factors, the benefits of ovulation induction and Assisted Reproductive Technology (ART) need to be weighed against the risks. It should be noted that pregnancy also carries an increased risk of thrombosis.
5.4 Ovarian Torsion
Ovarian torsion has been reported after treatment with gonadotropins, including GONAL-F. This may be related to OHSS, pregnancy, previous abdominal surgery, past history of ovarian torsion, previous or current ovarian cyst and polycystic ovaries. Early diagnosis and immediate detorsion limit damage to the ovary due to reduced blood supply.
5.5 Abnormal Ovarian Enlargement
In order to minimize the hazards associated with abnormal ovarian enlargement that may occur with GONAL-F therapy, individualize treatment and use the lowest effective dose [see Dosage and Administration (2.2, 2.3)]. Use of ultrasound monitoring of ovarian response and/or measurement of serum estradiol levels is important to minimize the risk of ovarian stimulation [see Warnings and Precautions (5.12)].
If the ovaries are abnormally enlarged on the last day of GONAL-F therapy, do not administer hCG in order to reduce the chance of developing Ovarian Hyperstimulation Syndrome (OHSS) [see Warnings and Precautions (5.2)]. Prohibit intercourse for women with significant ovarian enlargement after ovulation because of the danger of hemoperitoneum resulting from rupture of ovarian cysts [see Warnings and Precautions (5.2)].
5.6 Multi-fetal Gestation and Birth
Multi-fetal gestation and births have been reported with all gonadotropin therapy, including therapy with GONAL-F.
During clinical trials with GONAL-F, multiple births occurred in 20% of live births in women receiving therapy for ovulation induction and 35.1% of live births in women undergoing ART. Advise the woman and her partner of the potential risk of multi-fetal gestation and birth before beginning therapy with GONAL-F.
5.7 Embryofetal toxicity
The incidence of congenital malformations after some ART [specifically in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI)] may be slightly higher than after spontaneous conception. This slightly higher incidence is thought to be related to differences in parental characteristics (e.g., maternal age, maternal and paternal genetic background, sperm characteristics) and to the higher incidence of multi-fetal gestations after IVF or ICSI. There are no indications that the use of gonadotropins during IVF or ICSI is associated with an increased risk of congenital malformations.
5.8 Ectopic Pregnancy
Since infertile women undergoing ART often have tubal abnormalities, the incidence of ectopic pregnancy may be increased in women who become pregnant as a result of ART. Advise women who become pregnant following ART and have: abdominal/pelvic pain (particularly on one side); shoulder, neck or rectal pain; and nausea and vomiting to seek immediate medical attention. Confirm the presence of an intrauterine pregnancy early by β-hCG testing and transvaginal ultrasound.
5.9 Spontaneous Abortion
The risk of spontaneous abortion (miscarriage) is increased with gonadotropin products, including GONAL-F. However, causality has not been established. The increased risk may be a factor of the underlying infertility.
5.10 Ovarian Neoplasms
There have been infrequent reports of ovarian neoplasms, both benign and malignant, in women who have had multiple drug therapy for controlled ovarian stimulation, however, a causal relationship has not been established.
5.11 Laboratory Tests
In most instances, treatment of women with GONAL-F will result only in follicular recruitment and development. In the absence of an endogenous LH surge, hCG is given to trigger ovulation when monitoring of the woman indicates that sufficient follicular development has occurred. This may be estimated by ultrasound alone or in combination with measurement of serum estradiol levels. The combination of both ultrasound and serum estradiol measurement are useful for monitoring follicular growth and maturation, timing of the ovulatory trigger, detecting ovarian enlargement and minimizing the risk of the OHSS and multiple gestation.
The clinical confirmation of ovulation is obtained by direct or indirect indices of progesterone production as well as sonographic evidence of ovulation.
Direct or indirect indices of progesterone production:
- Urinary or serum luteinizing hormone (LH) rise
- A rise in basal body temperature
- Increase in serum progesterone
- Menstruation following a shift in basal body temperature
Sonographic evidence of ovulation:
- Collapsed follicle
- Fluid in the cul-de-sac
- Features consistent with corpus luteum formation
- Secretory endometrium
-
Acute Phase:
-
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed elsewhere in the labeling:
- Hypersensitivity Reactions and Anaphylaxis [see Warnings and Precautions (5.1)]
- Ovarian Hyperstimulation Syndrome [see Warnings and Precautions (5.2)]
- Pulmonary and Vascular Complication[see Warnings and Precautions (5.3)]
- Ovarian Torsion [see Warnings and Precautions (5.4)]
- Abnormal Ovarian Enlargement [see Warnings and Precautions (5.5)]
- Multi-fetal Gestation and Birth [see Warnings and Precautions (5.6)]
- Embryofetal Toxicity [see Warnings and Precautions (5.7)]
- Ectopic Pregnancy [see Warnings and Precautions (5.8)]
- Spontaneous Abortion [see Warnings and Precautions (5.9)]
- Ovarian Neoplasms [see Warnings and Precautions (5.10)]
6.1 Clinical Study Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trial of another drug and may not reflect the rates observed in practice.
Women:
The safety of GONAL-F was examined in four clinical trials that enrolled 691 women [two trials for ovulation induction (454 women) and two trials for ART (237 women)].
Induction of Ovulation
In a randomized, open-labeled, multicenter, active-controlled trial in oligo-anovulatory infertile women, conducted in the U.S., a total of 118 oligo-anovulatory infertile women were randomized to and underwent ovulation induction with GONAL-F versus a comparator urofollitropin. Adverse reactions occurring in at least 5.0% of women receiving GONAL-F are listed in Table 1.
Table 1: Common Adverse Reactions Reported at a Frequency of ≥ 5% in an U.S. Ovulation Induction Trial System Organ Class/Adverse Reactions GONAL-F
N=118* (288 treatment cycles†)
n‡ (%)Body as a Whole - General Pain 6 (5.1%) Central and Peripheral Nervous System Headache 12 (10.2%) Gastrointestinal System Abdominal Pain 9 (7.6%) Nausea 7 (5.9%) Flatulence 7 (5.9%) Reproductive, Female Intermenstrual Bleeding 6 (5.1) Ovarian Hyperstimulation 8 (6.8%) Ovarian Cyst 17 (14.4%) Development of Multiple Follicles as part of an Assisted Reproductive Technology (ART) Cycle
In a randomized, open-labeled, active-comparator trial conducted in the U.S., a total of 56 normal ovulatory infertile women were randomized and received GONAL-F versus a urofollitropin comparator as part of an ART [in vitro fertilization (IVF) or intracytoplasmic sperm injection cycle (ICSI)] cycle. All women received pituitary down-regulation with gonadotropin releasing hormone (GnRH) agonist before stimulation. Adverse Reactions occurring in at least 5.0% of women are listed in Table 2.
Table 2: Common Adverse Reactions Reported at a Frequency of ≥ 5% in an U.S. ART Trial System Organ Class/Adverse Reactions GONAL-F
(N=56*)
n† (%)Central and Peripheral Nervous System Headache 7 (12.5%) Gastrointestinal System Abdominal Pain 3 (5.4%) Nausea 4 (7.1%) Reproductive, Female Pelvic Pain 4 (7.1) Induction of Spermatogenesis:
The safety of GONAL-F for induction of spermatogenesis in men with primary or secondary hypogonadotropic hypogonadism was examined in 3 open-label, non-randomized, multi-center, multi-national, escalating dose clinical trials (Trials 1, 2 and 3) conducted in in 76 adult men (aged 16 to 48 years) with primary or secondary hypogonadotropic hypogonadism (defined as serum testosterone <100 ng/mL and low or normal FSH and LH) and azoospermia (sperm concentration <0.1×106/mL). Of the 76 men enrolled, 63 received treatment with GONAL-F.
During these trials, there was one serious adverse reaction of gynecomastia requiring surgical excision of breast tissue in a 50 year old man who received 9 months of therapy with Gonal-F. Pathology report showed gynecomastia with no atypia.
There were no discontinuations due to adverse reactions.
Adverse reactions reported in Trials 1, 2 and 3 by ≥2 patients during treatment with Gonal-f are shown in Table 3.
Table 3. Common Adverse Reactions in Men with Azoospermia and Primary or Secondary Hypogonadotropic Hypogonadism Receiving Gonal-F in Trials 1, 2 and 3 for Induction for Spermatogensis N=63
n (%)Acne 17 (27) Injection site pain 7 (11) Gynecomastia 4 (6) Seborrhea 3 (5) Fatigue 6 (10) Libido decreased 2 (3) 6.2 Postmarketing Experience
In addition to adverse events reported from clinical trials, the following adverse reactions have been reported during postmarketing use of GONAL-F. Because these reactions were reported voluntarily from a population of uncertain size, the frequency or a causal relationship to GONAL-F cannot be reliably determined.
Body as a Whole - General: Hypersensitivity reactions including anaphylaxis
Respiratory System: Asthma exacerbation
Vascular Disorders: Thromboembolism
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
GONAL-F is not indicated in pregnant women
The incidence of congenital malformations after some Assisted Reproductive Technology, specifically in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI)], may be slightly higher than that after spontaneous conception. This slightly higher incidence is thought to be related to differences in parental characteristics (e.g., maternal age, maternal and paternal genetic background, sperm characteristics) and to a higher incidence of multi-fetal gestations after IVF or ICSI. There is no human data that the use of gonadotropins (including GONAL-F) alone or as part of IVF or ICSI cycles, increases the risk of congenital malformations.
The risk of spontaneous abortion (miscarriage) is increased in women who have used gonadotropins products (including GONAL-F) to achieve pregnancy.
In animal studies, the continuous administration of recombinant human FSH during pregnancy resulted in a decrease in the number of viable fetuses and difficult and prolonged delivery. No teratogenic effect has been observed.
In the US general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Human Data
Data on a limited number of exposed pregnancies indicate no adverse reactions of gonadotropins on pregnancy, embryonal or fetal development, parturition or postnatal development following controlled ovarian stimulation.
Animal Data
Embryofetal development studies with recombinant human FSH in rats, where dosing occurred during organogenesis, showed a dose dependent increase in difficult and prolonged parturition in dams, and dose dependent increases in resorptions, pre- and post-implantation losses, and stillborn pups at doses representing 5 and 41 times the lowest clinical dose of 75 International Units based on body surface area. Pre-/post-natal development studies with recombinant human FSH in rats, where dosing occurred from mid-gestation through lactation, showed difficult and prolonged parturition in all dams dosed at 41 times the lowest clinical dose of 75 International Units based on body surface area, along with maternal death and stillborn pups associated with the difficult and prolonged parturition. This toxicity was not observed in dams and offspring dosed at a level 5 times the lowest clinical dose of 75 International Units based on body surface area.
8.2 Lactation
There are no data on the presence of GONAL-F in human milk, the effects on the breastfed infant, or the effects on milk production. Because the secretion of prolactin during lactation can result in inadequate response to ovarian stimulation, advise women not to breast feed during treatment with GONAL-F.
8.3 Females and Males of Reproductive Potential
Because GONAL-F is not indicated in pregnant women, verify a negative pregnancy test before administering GONAL-F to a woman [see Dosage and Administration (2.3, 2.4)].
-
10 OVERDOSAGE
Ovarian hyperstimulation syndrome (OHSS) and multiple gestations have been observed in women with GONAL-F overdosage [see Warnings and Precautions (5.2,5.6)].
-
11 DESCRIPTION
Follitropin alfa, a gonadotropin [human follicle stimulating hormone (hFSH)], is a glycoprotein hormone produced by recombinant DNA technology in a Chinese Hamster Ovary (CHO) cell line. It has a dimeric structure containing two glycoprotein subunits (alpha and beta). The alpha and beta subunits have 92 and 111 amino acids, respectively, and their primary and tertiary structures are indistinguishable from those of human follicle stimulating hormone. The molecular weight is approximately 31 kDa (14 kDa for alpha subunit and 17 kDa for beta subunit).
GONAL-F (follitropin alfa) for injection is a sterile, lyophilized powder intended for subcutaneous injection after reconstitution.
Each multiple-dose vial of GONAL-F containing either 450 International Units (33 mcg) or 1050 International Units (77 mcg) follitropin alfa and the inactive ingredients dibasic sodium phosphate (0.89 mg), monobasic sodium phosphate (0.39 mg), and sucrose (30 mg). Phosphoric acid and/or sodium hydroxide may be used prior to lyophilization for pH adjustment. After reconstitution with supplied 1 mL of with Bacteriostatic Water for Injection (0.9% benzyl alcohol), USP, the resultant concentration is 600 IU/mL with a pH of approximately 6.5 to 7.5.
Under current storage conditions, GONAL-F may contain up to 10% of oxidized follitropin alfa.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
GONAL-F stimulates ovarian follicular growth in women who do not have primary ovarian failure. In order to bring about final maturation of the follicle and ovulation in the absence of an endogenous LH surge, human chorionic gonadotropin (hCG) must be given, following the administration of GONAL-F, when monitoring of the patient indicates that sufficient follicular development is achieved.
GONAL-F stimulates spermatogenesis in men with hypogonadotropic hypogonadism when administered with hCG.
12.2 Pharmacodynamics
Serum inhibin, estradiol, and total follicular volume responded as a function of time, with pronounced inter-woman variability in healthy volunteers administered GONAL-F. Pharmacodynamic effect lagged behind FSH serum concentration. Serum inhibin levels responded with the least delay and declined rapidly after discontinuation of GONAL-F. Follicular growth was most delayed and continued even after discontinuation of GONAL-F, and after serum FSH levels had declined. Maximum follicular volume correlated better with inhibin and estradiol peak levels than with FSH concentration. Inhibin rise was an early index of follicular development.
FSH serum levels following fixed (during the first five days) and then adjusted doses of GONAL-F were found to be poor predictors of follicular growth rate. High pre-treatment serum FSH levels may predict lower follicular growth rates.
Inhibin levels reached a plateau during the entire administration period and then returned to baseline despite high inter-male variation and the absence of down-regulation in healthy male volunteers administered GONAL-F.
12.3 Pharmacokinetics
Single dose and state-state pharmacokinetics of follitropin alfa were determined following subcutaneous administration of GONAL-F to healthy, down-regulated female volunteers, healthy adult male volunteers, and pituitary down-regulated women undergoing in vitro fertilization and embryo transfer (IVF/ET). The pharmacokinetic parameters of follitropin alfa following subcutaneous administration of GONAL-F are presented in Table 4.
Table 4: Pharmacokinetic Parameters (mean ± SD) of Follitropin Alfa Population Female Male Healthy Female Volunteers IVF/ET Patients Healthy Male Volunteers Dose (IU) Single Dose
(150 IU)Multiple Dose
(7 × 150 IU)Multiple Dose
(5 × 225 IU),Single Dose
(225 IU)Multiple Dose
(7 × 225 IU)AUC=area under the concentration-time curve; CL/F=apparent clearance; Cmax=peak serum concentration; Tmax=time of Cmax; t1/2=half-life; Vd=volume of distribution - *
- Steady-state AUC144 hr-168 hr (after the 7th daily subcutaneous dose)
- †
- Follitropin alfa metabolism has not been studied in humans.
- ‡
- The elimination rate of follitropin alfa following subcutaneous administration is dependent on the absorption rate.
- §
- The apparent clearance was comparable to that in healthy volunteers.
General Information AUC (IU*hr/L) 176 ± 87 187 ± 61* --- 220 ± 109 186 ± 23* Cmax (IU/L) 3 ± 1 9 ± 3 --- 2.5 ± 0.8 8.3 ± 0.9 Absorption Absolute Bioavailability (%) 66 ± 39 --- --- --- Tmax (hr) 16 ± 10 8 ± 6 --- 20 ± 14 10.7 ± 6.7 Distribution Apparent Vd (L) ------ --- 10 ± 3 --- --- Elimination† t1/2 terminal (hr)‡ 24 ± 11 24 ± 8 32 41 ± 14 32 ± 4 CL/F (L/hr)§ ------ --- 0.7 ± 0.2 0.86 ± 0.48 0.90 ± 0.12 -
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long- term studies in animals have not been performed to evaluate the carcinogenic potential of GONAL-F. However, follitropin alfa showed no mutagenic activity in a series of tests performed to evaluate its potential genetic toxicity including, bacterial and mammalian cell mutation tests, a chromosomal aberration test and a micronucleus test.
Impaired fertility has been reported in rats, exposed to pharmacological doses of follitropin alfa (greater than or equal to 40 International Units per kg per day, greater than or equal to 5 times the lowest clinical dose of 75 International Units) for extended periods, through reduced fecundity.
-
14 CLINICAL STUDIES
14.1 Induction of Ovulation
The safety and efficacy of GONAL-F were examined in a randomized, open-label, multicenter, active-controlled trial conducted in the U.S in oligo-anovulatory infertile women. Women were randomized to GONAL-F , administered subcutaneously, or a comparator urofollitropin product, administered intramuscularly.
The primary efficacy parameter was the ovulation rate. Two hundred and thirty-two women received treatment for up to three cycles with GONAL-F (118 women ) or urofollitropin (114 women).
Ovulation results for women who received treatment with GONAL-F in at least one cycle are summarized in Table 5.
Table 5: Cumulative Ovulation and Clinical Pregnancy Rates in Induction of Ovulation Trial Cycle Gonal-F (n=118) Cumulative* Percent Ovulation Cumulative* Clinical Pregnancy† Rate - *
- Cumulative rates were determined per woman over cycles 1, 2, and 3
- †
- Clinical pregnancy was defined as a pregnancy for which a fetal sac (with or without heart activity) was visualized by ultrasound on day 34-36 after hCG administration
- ‡
- Non-inferior to comparator recombinant human FSH based on a two-sided 95% confidence interval, intent-to-treat analysis.
- §
- Secondary efficacy outcomes. The trial was not powered to demonstrate differences in these outcomes.
Cycle 1 58%‡ 13%§ Cycle 2 72%§ 25%§ Cycle 3 81%§ 37§ For the 44 woman in the GONAL-F group who achieved clinical pregnancy, 22.7 % did not reach a term pregnancy, 63.6% had singleton births and 13.7% had multiple births.
An additional randomized, open-label, multinational, multicenter trial, active-comparator trial was conducted in oligo-anovulatory infertile women who failed to ovulate or conceive following adequate clomiphene citrate therapy. Results for the primary efficacy outcome of cumulative percent ovulation at Cycle 1 were similar to those presented in Table 5 for the U.S. ovulation induction trial.
14.2 Development of Multiple Follicles as part of an Assisted Reproductive Technology (ART) Cycle:
The efficacy of GONAL-F in ART, was evaluated in a randomized,open-label, multicenter, active-controlled trial conducted ith the U.S, in ovulatory, infertile women undergoing stimulation of multiple follicles for In Vitro Fertilization (IVF) and Embryo Transfer (ET). All women received a gonadotrophin releasing hormone (GnRH) agonist for pituitary down-regulation before randomization and administration of GONAL-F (n=56) or a comparator urofollitropin product (n=58). The primary efficacy endpoint was the number of mature pre-ovulatory follicles on the day of hCG administration. The trial was not powered to demonstrate differences in secondary outcomes.
Treatment outcomes for a single IVF cycle with controlled stimulation with GONAL-F are summarized in Table 6.
Table 6: Treatment Outcomes with GONAL-F in an In Vitro Fertilization Trial in Ovulatory Women Gonal-F (n=56) - *
- Primary efficacy outcome
- †
- Secondary efficacy outcomes. The trial was not powered to demonstrate differences in these outcomes.
- ‡
- Clinical pregnancy was defined as a pregnancy for which a fetal sac (with or without heart activity) was visualized by ultrasound on day 34-36 after hCG administration.
Mean number of follicles ≥ 14mm diameter on day of hCG* (n=50) 7.2 Mean number of oocytes recovered per patient† (n=49) 9.3 Mean Serum E2 (pg/mL) on day of hCG† (n=46) 1221 Mean treatment duration in days (range)† (n=56) 10.1 (5-15) Clinical pregnancy‡rate per attempt† (n=56) 20% Clinical pregnancy‡ rate per embryo transfer† (n=47) 23% For the 11 woman in the GONAL-F group who achieved clinical pregnancy, 36.3% did not reach a term pregnancy, 36.3% had singleton births and 27.3% had multiple births.
An additional randomized, open-label, multinational, multicenter study in ovulatory infertile women was conducted in non-U.S. countries. Women were randomized to receive either GONAL-F by subcutaneous administration (60 women) or urofollitropin by intramuscular administration (63 women) after down-regulation of the pituitary with a GnRH agonist. The primary efficacy parameter was the number of mature pre-ovulatory follicles on the day of hCG administration. Results over a single IVF cycle for the primary efficacy outcome of mature pre-ovulatory follicles on the day of hCG administration were similar to the primary efficacy results presented in Table 6 for the U.S.ART trial.
14.3 Induction of Spermatogenesis in Males
The efficacy of GONAL-F administered concomitantly with human chorionic gonadotropin (hCG) for induction of spermatogenesis in men with hypogonadotropic hypogonadism was established in three open-label, uncontrolled, non-randomized, multi-center, multi-national, escalating dose clinical trials (Trials 1, 2 and 3) conducted in 78 adult men (aged 16 to 48 years) with primary or secondary hypogonadotropic hypogonadism (defined as serum testosterone <100 ng/mL and low or normal FSH and LH) and azoospermia (sperm concentration <0.1×106/mL). Men were required at study entry to have normal serum cortisol and prolactin levels and be euthyroid. Men less than 21 years of age were required to have either confirmed anosmia or documented bone age >15 years to be eligible for study participation. Enrolled men received three to six months of pretreatment with hCG injection to normalize serum testosterone levels, followed by 18 months of treatment with GONAL-F and hCG.
Of the 78 men enrolled in the trials, 63 men were treated with GONAL-F and hCG.
Characteristics of the trial populations are shown in Table 7.
Table 7. Trial Population Characteristics in Trials 1, 2 and 3 Trial 1 N=32 Trial 2 N=10 Trial 3 N=36 Median age (range) (years) 26 (16-48) 37 (26-48) 30 (20-44) Race n(%) Caucasian 31 (97) 7 (70) 31 (86) Asian 1 (3) 3 (30) 3 (8) African-American 0 0 0 Other 0 0 2 (6) Prior treatment with gonadotropin (FSH) or GnRH* agonist† (%) 0 5 (50) 4 (11) Mean (SD) testis volume (mL)‡ 2 (1) 5 (3) 4 (1) N(%) with anosmia (i.e. diagnosis of Kallmann's syndrome) 12 (37) 2 (20) 13 (36) The primary efficacy measure in all trials was the proportion of men achieving a sperm density ≥ 1.5 × 106/mL during treatment with Gonal-F. Pregnancy (clinical and chemical) in partners of men desiring fertility was a secondary endpoint. Efficacy results in men who received at least one dose of Gonal-F and had at least one follow-up assessment are summarized in Table 8 and Table 9.
Table 8: Proportion of Men Receiving Gonal-F Who Achieved a Sperm Density ≥ 1.5 × 106/mL Trial 1 (n=26) Trial 2 (n=8) Trial 3 (n=29) Sperm Concentration ≥ 1.5 × 106/mL Yes 12 (46.2%) 5 (62.5%) 20 (80%) No 14 (53.8%) 3 (37.5%) 5 (20%) Missing 4 95% Confidence Interval (26.6% - 66.6%) (24.5% - 91.5%) (40.7% - 82.8%) Table 9: Pregnancy Outcome in Partners of Men Desiring Fertility Trial 1 (n=7)* Trial 2 (n=10)* Trial 3 (n=26)*, - *
- N reflects number of partners desiring pregnancy who had a partner at the time of enrollment, as not all enrolled men sought fertility
Pregnancy 6 (86%) 3 (30%) 5 (19%) Pregnancy not reaching term 1 (14%) 1 (10%) 2 (8%) Single full-term live births 5 (71%) 2 (20%) 3 (11%) The time to achievement of sperm density ≥1.5 × 106/mL is summarized in Table 10.
Table 10: Time to Achievement of Sperm Density ≥ 1.5 × 106/ mL in Men Receiving Gonal-F Trial 1 (n=26) Trial 2 (n=8) Trial 3 (n=29) Number (%) of Men Achieving Sperm Concentration 22 (76) n 12 (46) 5 (62) Time (Months) to Sperm Concentration ≥ 1.5 × 106/mL Median 12.4 9.1 9 Range (2.7 – 18.1) (8.8 – 11.7) (2.8 – 18.2) -
16 How Supplied/Storage and Handling
16.1 How Supplied
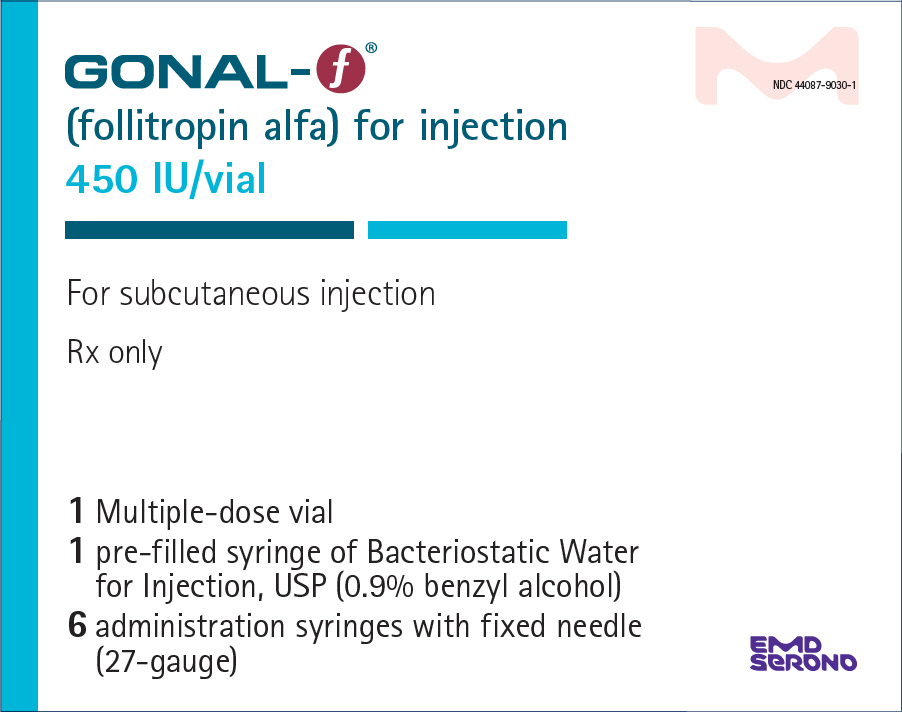
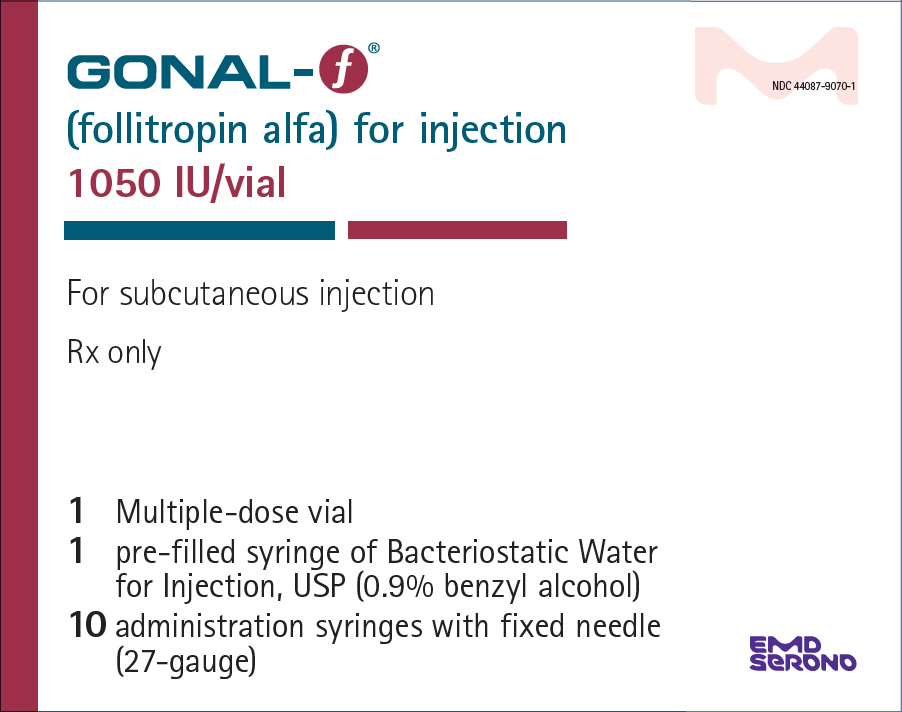
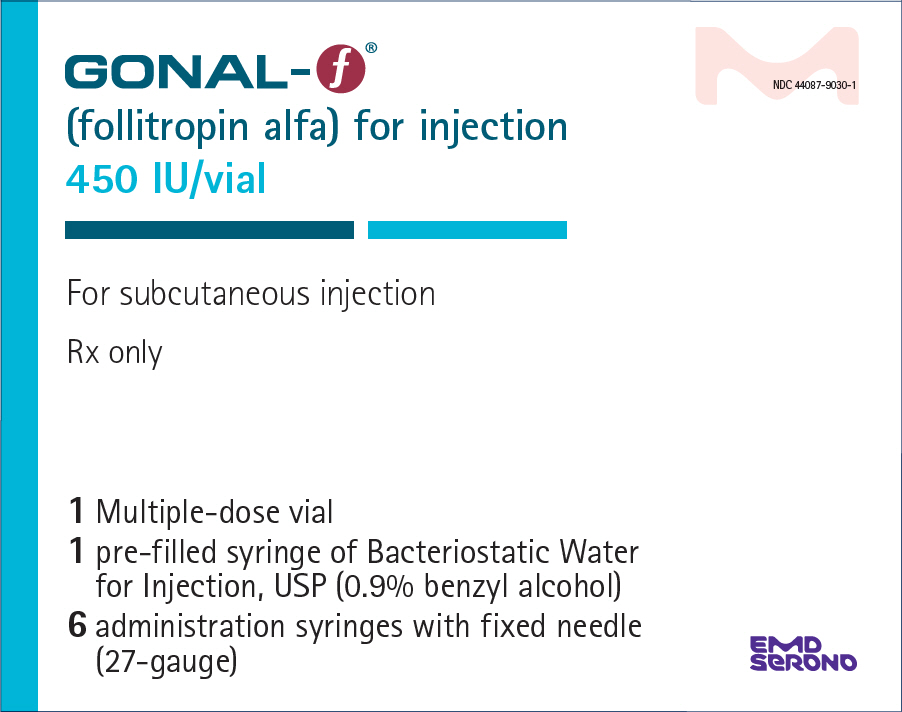
GONAL-F (follitropin alfa) for injection is supplied as a sterile, white lyophilized powder in multiple-dose vials of either 450 International Units per vial or 1050 International Units per vial.
The following package presentations are available:
NDC 44087-9030-1 - One multiple-dose vial of GONAL-F 450 International Units, one-1 mL prefilled diluent syringe of Bacteriostatic Water for Injection, USP (0.9% benzyl alcohol), and six adminstration syringes calibrated in FSH Units (IU FSH) with a fixed 27-gauge × 0.5-inch needle.
NDC 44087-9070-1 - One multiple-dose vial GONAL-F of 1050 International Units, one-2 mL prefilled diluent syringe of Bacteriostatic Water for Injection, USP (0.9% benzyl alcohol), and ten adminstration syringes calibrated in FSH Units (IU FSH) with a fixed 27-gauge × 0.5-inch needle.
16.2 Storage and Handling
Store vials refrigerated between 2°C to 8°C (36°F to 46°F) or at room temperature between 20°C to 25°C (68°F to 77°F).
Store reconstituted solution refrigerated between 2°C to 8°C (36°F to 46°F) or at room temperature between 20°C to 25°C (68°F to 77°F) and discard unused portion after 28 days. Protect from light [see Dosage and Administration (2.2)].
-
17 PATIENT COUNSELING INFORMATION
Advise women and men to read the FDA-approved patient labeling (Patient Information and Instructions for Use)
Hypersensitivity Reactions and Anaphylaxis
Advise women and men to discontinue GONAL-F and seek immediate medical attention if any signs or symptoms of a hypersensitivity reaction occur [see Warnings and Precautions (5.1)].
Ovarian Hyperstimulation Syndrome
Inform women regarding the risks of OHSS [see Warnings and Precautions (5.2] and OHSS-associated conditions including pulmonary and vascular complications [see Warnings and Precautions (5.3)] and ovarian torsion [see Warnings and Precautions (5.4)] with the use of GONAL-F. Advise women to seek medical attention if any of these conditions occur.
Abnormal Ovarian Enlargement
Inform women regarding the hazards associated with abnormal ovarian enlargement that may occur with GONAL-F therapy. If the ovaries are abnormally enlarged on the last day of GONAL-F therapy, inform women not to administer hCG and to avoid intercourse [see Warnings and Precautions (5.5)].
Multi-fetal Gestation and Birth
Advise the woman and her partner of the potential risk of multi-fetal gestation and birth before beginning therapy withGONAL-F [see Warnings and Precautions (5.6)].
Embryofetal Toxicity
Inform women that the incidence of congenital malformations (birth defects) after some Assisted Reproductive Technology [(ART) specifically in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI)] may be slightly higher than after spontaneous conception [see Warnings and Precautions (5.7)].
Ectopic Pregnancy
Inform women undergoing ART that the incidence of ectopic pregnancy may be increased with these procedures, particularly for women with tubal abnormalities. Advise women who become pregnant and have: abdominal/pelvic pain (particularly on one side); shoulder, neck or rectal pain; and nausea and vomiting to seek immediate medical attention [see Warnings and Precautions (5.8)].
Spontaneous Abortion
Inform women that the risk of spontaneous abortion (miscarriage) is increased with gonadotropin products (including GONAL-F). However, causality has not been established. The increased risk may be a factor of the underlying infertility [see Warnings and Precautions (5.9)].
Lactation
Advise women not to breastfeed because the secretion of prolactin during lactation can result in inadequate response to ovarian stimulation with Gonal-F [see Use in Specific Populations (8.2)].
Dosing and Use of GONAL-F Multi Dose
Instruct women and men on the correct usage and dosing of GONAL-F [see Dosage and Administration (2.3, 2.4 2.5)]. Caution against changing the dosage or the schedule of administration unless instructed to do so by a healthcare provider.
Duration and Necessary Monitoring in Patients Undergoing Therapy with GONAL-F
Prior to beginning therapy with GONAL-F, inform women and men about the time commitment and monitoring procedures necessary for treatment [see Dosage and Administration (2.3, 2.4, 2.5) and Warnings and Precautions (5.11)].
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - Kit Carton - 450 IU
- PRINCIPAL DISPLAY PANEL - Kit Carton - 1050 IU
-
INGREDIENTS AND APPEARANCE
GONAL-F
follitropin alfa kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:44087-9030 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:44087-9030-1 1 in 1 CARTON 03/25/2004 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL 1 mL Part 2 1 SYRINGE 1 mL Part 1 of 2 GONAL-F
follitropin alfa injection, powder, lyophilized, for solutionProduct Information Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FOLLITROPIN (UNII: 076WHW89TW) (FOLLITROPIN - UNII:076WHW89TW) FOLLITROPIN 450 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) 30 mg in 1 mL SODIUM PHOSPHATE, DIBASIC, DIHYDRATE (UNII: 94255I6E2T) 1.11 mg in 1 mL SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE (UNII: 593YOG76RN) 0.45 mg in 1 mL PHOSPHORIC ACID (UNII: E4GA8884NN) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 mL in 1 VIAL; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA020378 03/25/2004 Part 2 of 2 BACTERIOSTATIC WATER
water and benzyl alcohol injection, solutionProduct Information Route of Administration SUBCUTANEOUS Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 1 mL in 1 mL BENZYL ALCOHOL (UNII: LKG8494WBH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 mL in 1 SYRINGE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA020378 03/25/2004 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA020378 03/25/2004 GONAL-F
follitropin alfa kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:44087-9070 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:44087-9070-1 1 in 1 CARTON 03/25/2004 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL 2 mL Part 2 1 SYRINGE 2 mL Part 1 of 2 GONAL-F
follitropin alfa injection, powder, lyophilized, for solutionProduct Information Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FOLLITROPIN (UNII: 076WHW89TW) (FOLLITROPIN - UNII:076WHW89TW) FOLLITROPIN 1050 [iU] in 2 mL Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) 30 mg in 2 mL SODIUM PHOSPHATE, DIBASIC, DIHYDRATE (UNII: 94255I6E2T) 1.11 mg in 2 mL SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE (UNII: 593YOG76RN) 0.45 mg in 2 mL PHOSPHORIC ACID (UNII: E4GA8884NN) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 2 mL in 1 VIAL; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA020378 03/25/2004 Part 2 of 2 BACTERIOSTATIC WATER
water and benzyl alcohol injection, solutionProduct Information Route of Administration SUBCUTANEOUS Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 2 mL in 2 mL BENZYL ALCOHOL (UNII: LKG8494WBH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 2 mL in 1 SYRINGE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA020378 03/25/2004 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA020378 03/25/2004 Labeler - EMD Serono, Inc. (088514898)