CLINIMIX- isoleucine, leucine, valine, lysine, methionine, phenylalanine, threonine, tryptophan, arginine, histidine, alanine, glycine, proline, serine, tyrosine, sodium acetate, dibasic potassium phosphate, sodium chloride, magnesium chloride, dextrose monohydrate, calcium chloride solution
Baxter Healthcare Corporation
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
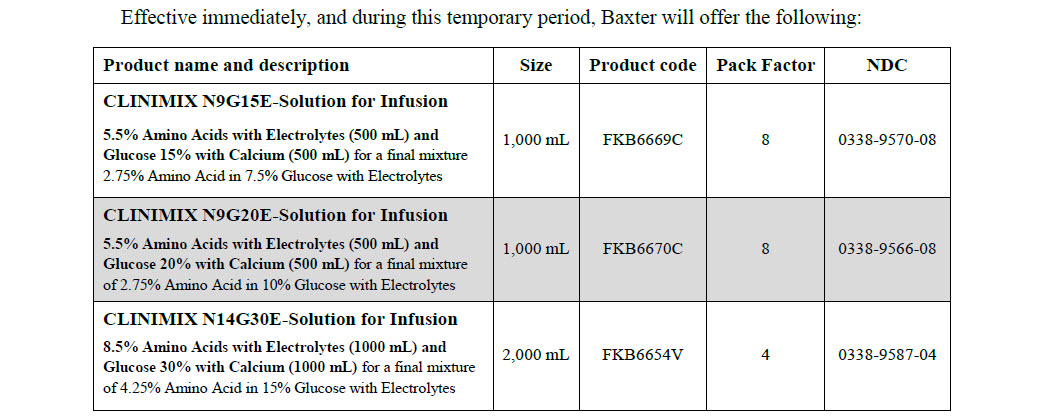
Clinimix, solution for infusion
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
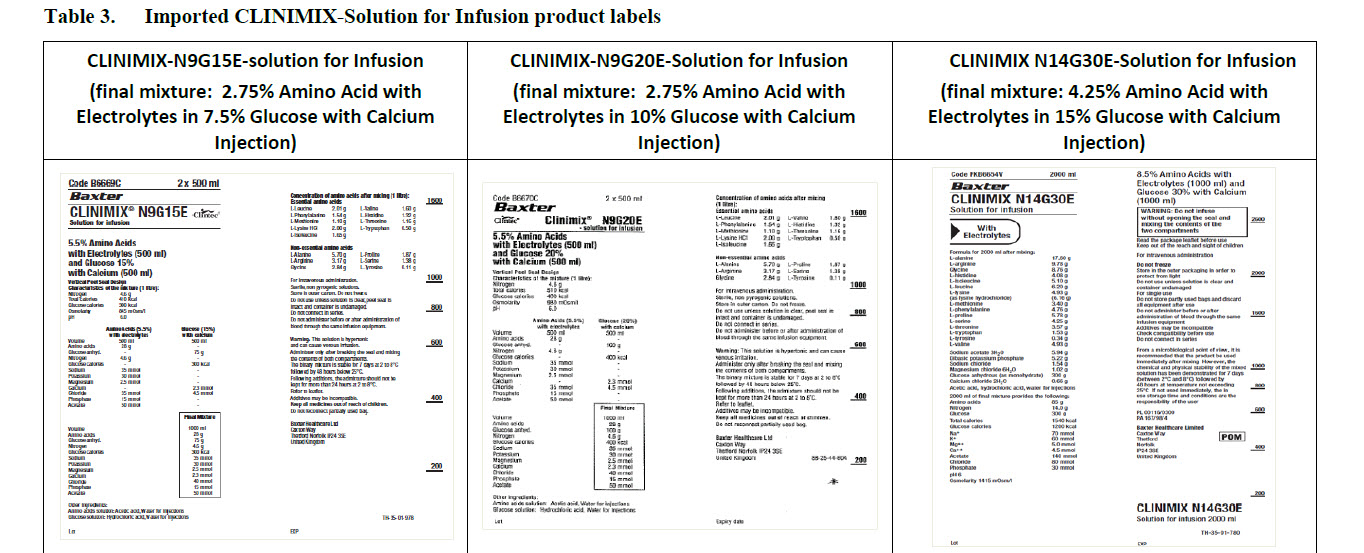
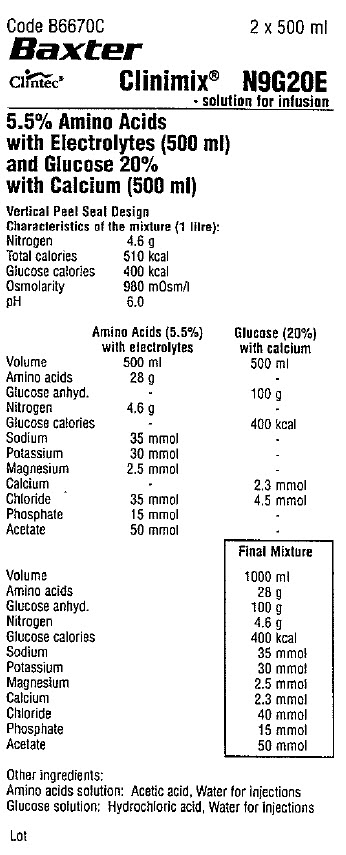
Code B6670C 2 x 500 ml
Baxter Logo
Clintec Logo Clinimix® N9G20E
- solution for infusion
5.5% Amino Acids
with Electrolytes (500 ml)
and Glucose 20%
with Calcium (500 ml)
Vertical Peel Seal Design
Characteristics of the mixture (1 litre):
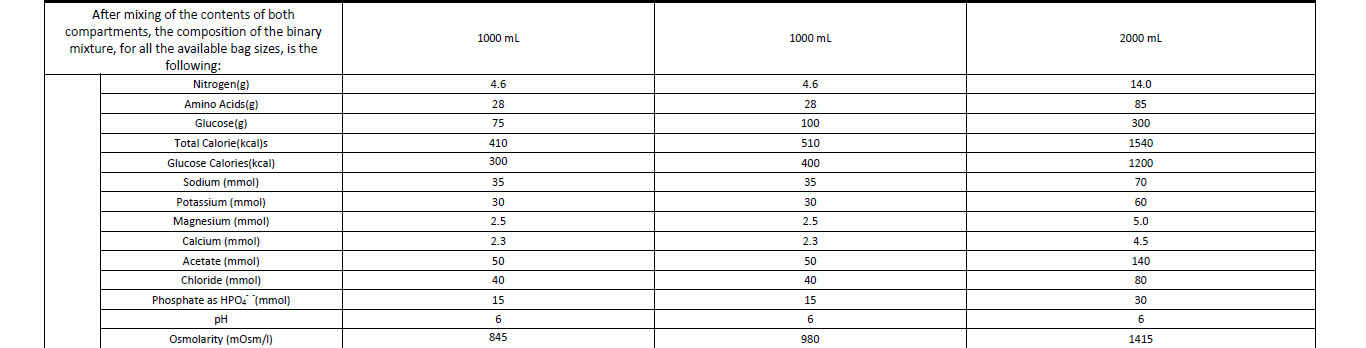
Nitrogen 4.6 g
Total calories 510 kcal
Glucose calories 400 kcal
Osmolarity 980 mOsm/1
pH 6.0
Amino Acids (5.5%)
with electrolytes
Volume 500 ml
Amino acids 28 g
Glucose anhyd. -
Nitrogen 4.6 g
Glucose calories -
Sodium 35 mmol
Potassium 30 mmol
Magnesium 2.5 mmol
Calcium -
Chloride 35 mmol
Phosphate 15 mmol
Acetate 50 mmol
Glucose (20%)
with calcium
Volume 500 ml
Amino acids -
Glucose anhyd. 100 g
Nitrogen -
Glucose calories 400 kcal
Sodium -
Potassium -
Magnesium -
Calcium 2.3 mmol
Chloride 4.5 mmol
Phosphate -
Acetate -
Final Mixture
Volume 1000 ml
Amino acids 28 g
Glucose anhyd. 100 g
Nitrogen 4.6 g
Glucose calories 400 kcal
Sodium 35 mmol
Potassium 30 mmol
Magnesium 2.5 mmol
Calcium 2.3 mmol
Chloride 40 mmol
Phosphate 15 mmol
Acetate 50 mmol
Other ingredients:
Amino acids solution: Acetic acid, Water for injections
Glucose solution: Hydrochloric acid, Water for injections
Lot
Concentration of amino acids after mixing
(1 litre):
Essential amino acids
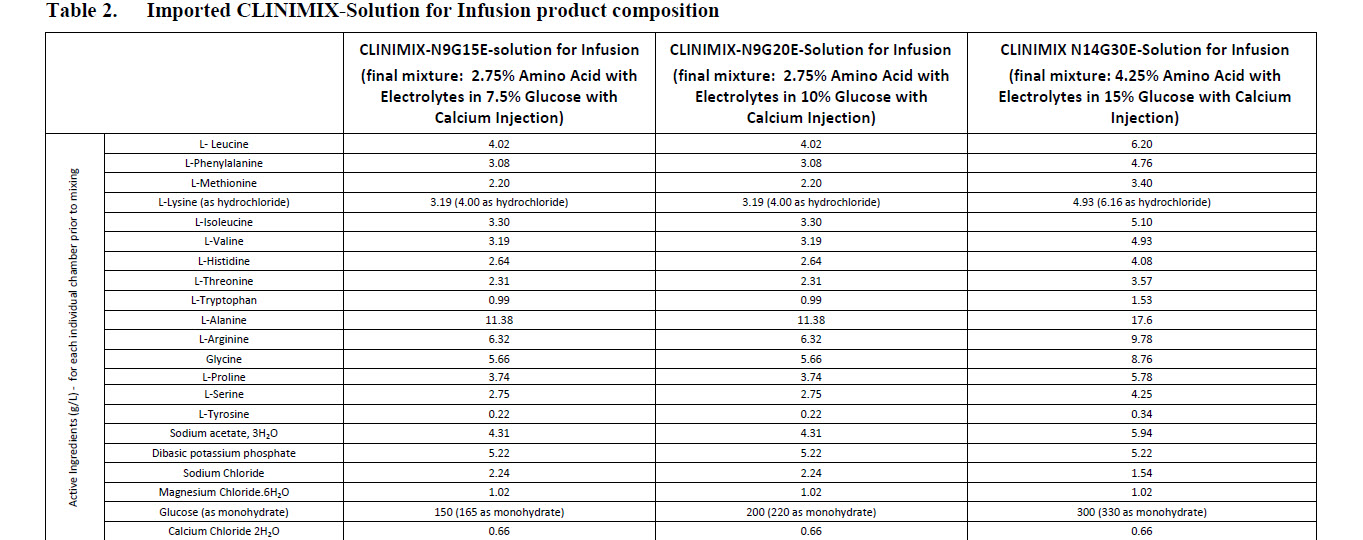
L-Leucine 2.01 g L-Valine 1.60 g
L-Phenylalanine 1.54 g L-Histidine 1.32 g
L-Methionine 1.10 g L-Threonine 1.16 g
L-Lysine HCI 2.00 g L-Tryptophan 0.50 g
L-Isoleucine 1.65 g
Non-essenlial amino acids
L-Alanine 5.70 g L-Proline 1.87 g
L-Arginine 3.17 g L-Serine 1.38 g
Glycine 2.84 g L-Tyrosine 0.11 g
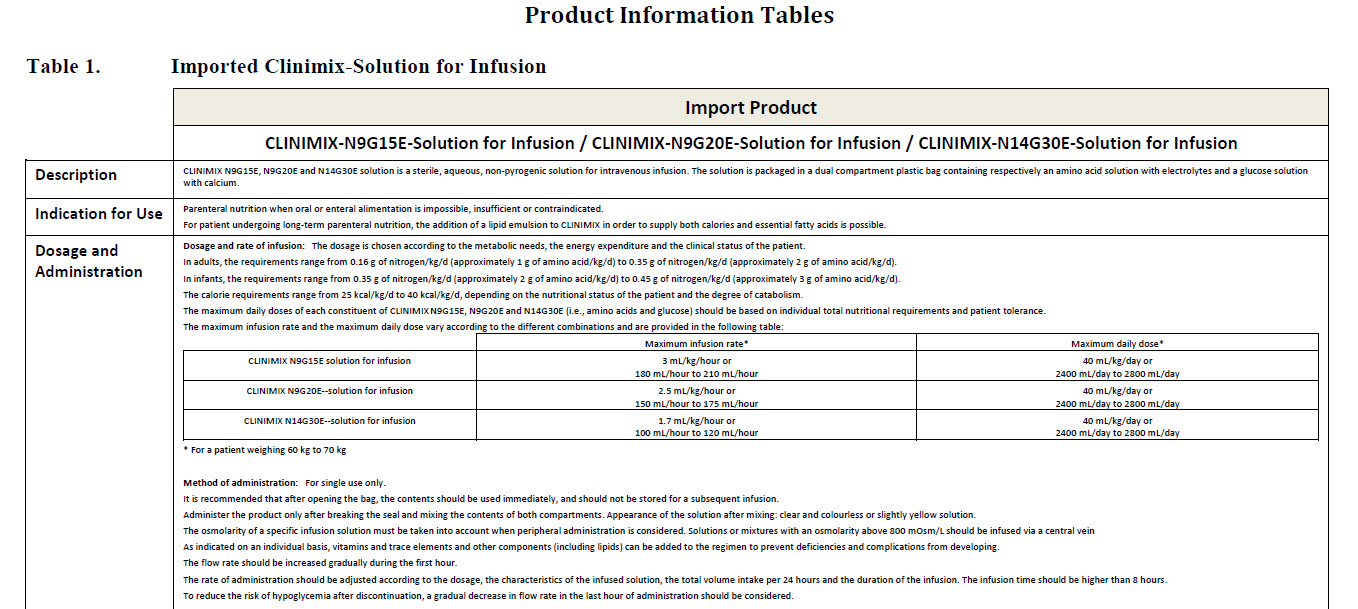
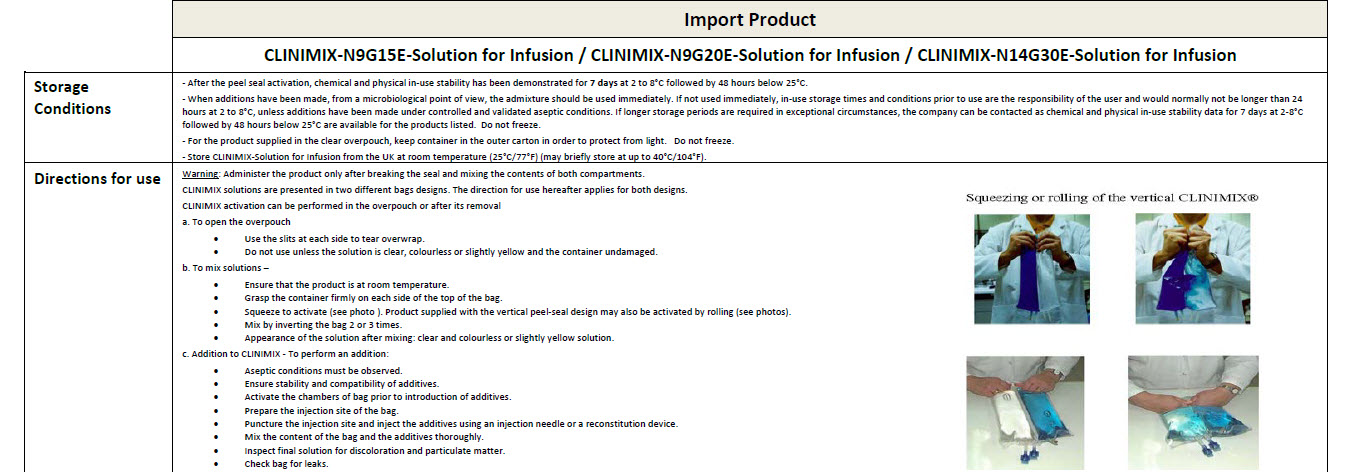
For intravenous administration.
Sterile, non pyrogenic solutions.
Store in outer carton. Do not freeze.
Do not use unless solution is clear, peel seal is
intact and container is undamaged.
Do not connect in series.
Do not administer before or after administration of
blood through the same infusion equipment.
Warning: This solution is hypertonic and can cause
venous irritation.
Administer only after breaking the seal and mixing
the contents of both compartments.
The binary mixture is stable for 7 days at 2 to 8°C
followed by 48 hours below 25°C.
Following additions, the admixture should not be
kept for more than 24 hours at 2 to 8°C.
Refer to leaflet.
Additives may be incompatible.
Keep all medicines out of reach of children.
Do not reconnect partially used bag.
Baxter Healthcare Ltd
Caxton Way
Thetford Norfolk IP24 3SE
United Kingdom
88-25-44-604
Expiry date
1600
1000
800
600
400
200
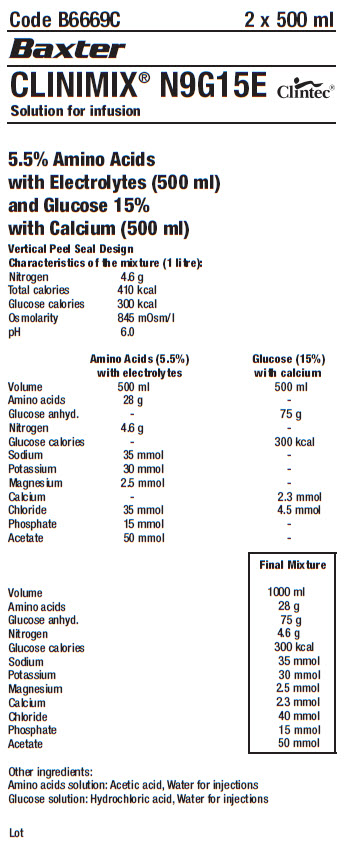
Code B6669C
2 x 500 ml
Baxter Logo
CLINIMIX® N9G15E
Solution for infusion
Clintec Logo
5.5% Amino Acids
with Electrolytes (500 ml)
and Glucose 15%
with Calcium (500 ml)
Vertical Peel Seal Design
Characteristics of the mixture (1 litre):
Nitrogen 4.6 g
Total calories 410 kcal
Glucose calories 300 kcal
Osmolarity 845 mOsm/l
pH 6.0
-
Amino Acids (5.5%)
with electrolytes
Volume 500 ml
Amino acids 28 g
Glucose anhyd. -
Nitrogen 4.6 g
Glucose calories -
Sodium 35 mmol
Potassium 30 mmol
Magnesium 2.5 mmol
Calcium -
Chloride 35 mmol
Phosphate 15 mmol
Acetate 50 mmol
Glucose (15%)
with calcium
Volume 500 ml
Amino acids -
Glucose anhyd. 75 g
Nitrogen -
Glucose calories 300 kcal
Sodium -
Potassium -
Magnesium -
Calcium 2.3 mmol
Chloride 4.5 mmol
Phosphate -
Acetate -
Final Mixture
- Volume 1000 ml
Amino acids 28 g
Glucose anhyd. 75 g
Nitrogen 4.6 g
Glucose calories 300 kcal
Sodium 35 mmol
Potassium 30 mmol
Magnesium 2.5 mmol
Calcium 2.3 mmol
Chloride 40 mmol
Phosphate 15 mmol
Acetate 50 mmol
Other ingredients:
Amino acids solution: Acetic acid, Water for injections
Glucose solution: Hydrochloric acid, Water for injections
Lot
Concentration of amino acids after mixing (1 litre):
Essential amino acids
- L-Leucine 2.01 g L-Valine 1.60 g
L-Phenylalanine 1.54 g L-Histidine 1.32 g
L-Methionine 1.10 g L-Threonine 1.16 g
L-Lysine HCl 2.00 g L-Tryptophan 0.50 g
L-Isoleucine 1.65 g
Non-essential amino acids
L-Alanine 5.70 g L-Proline 1.87 g
L-Arginine 3.17 g L-Serine 1.38 g
Glycine 2.84 g L-Tyrosine 0.11 g
For intravenous administration.
Sterile, non pyrogenic solutions.
Store in outer carton. Do not freeze.
Do not use unless solution is clear, peel seal is
intact and container is undamaged.
Do not connect in series.
Do not administer before or after administration of
blood through the same infusion equipment.
Warning: This solution is hypertonic
and can cause venous irritation.
Administer only after breaking the seal and mixing
the contents of both compartments.
The binary mixture is stable for 7 days at 2 to 8°C
followed by 48 hours below 25°C.
Following additions, the admixture should not be
kept for more than 24 hours at 2 to 8°C.
Refer to leaflet.
Additives may be incompatible.
Keep all medicines out of reach of children.
Do not reconnect partially used bag.
Baxter Healthcare Ltd
Caxton Way
Thetford Norfolk IP24 3SE
United Kingdom
TH-35-01-978
EXP
1600
1000
800
600
400
200
Code FKB6654V 2000 ml
Baxter Logo
CLINIMIX N14G30E
Solution for infusion
With Electrolytes
Formula for 2000 ml after mixing:
L-alanine 17.60 g
L-arginine 9.78 g
Glycine 8.76 g
L-histidine 4.08 g
L-isoleucine 5.10 g
L-leucine 6.20 g
L-lysine 4.93 g
(as lysine hydrochloride) (6.16 g)
L-methionine 3.40 g
L-phenylalanine 4.76 g
L-proline 5.78 g
L-serine 4.25 g
L-threonine 3.57 g
L-tryptophan 1.53 g
L-tyrosine 0.34 g
L-valine 4.93 g
Sodium acetate 3H2O 5.94 g
Dibasic potassium phosphate 5.22 g
Sodium chloride 1.54 g
Magnesium chloride 6H2O 1.02 g
Glucose anhydrous (as monohydrate) 300 g
Calcium chloride 2H2O 0.66 g
Acetic acid, hydrochloric acid, water for injections
2000 ml of final mixture provides the following:
Amino acids 85 g
Nitrogen 14.0 g
Glucose 300 g
Total calories 1540 kcal
Glucose calories 1200 kcal
Na+ 70 mmol
K+ 60 mmol
Mg++ 5.0 mmol
Ca++ 4.5 mmol
Acetate 140 mmol
Chloride 80 mmol
Phosphate 30 mmol
pH 6
Osmolarity 1415 mOsm/l
8.5% Amino Acids with
Electrolytes (1000 ml) and
Glucose 30% with Calcium
(1000 ml)
WARNING: Do not infuse
without opening the seal and
mixing the contents of the
two compartments
Read the package leaflet before use
Keep out of the reach and sight of children
For intravenous administration
Do not freeze
Store in the outer packaging in order to
protect from light
Do not use unless solution is clear and
container undamaged
For single use
Do not store partly used bags and discard
all equipment after use
Do not administer before or after
administration of blood through the same
infusion equipment
Additives may be incompatible
Check compatibility before use
Do not connect in series
From a microbiological point of view, it is
recommended that the product be used
immediately after mixing However, the
chemical and physical stability of the mixed
solution has been demonstrated for 7 days
(between 2°C and 8°C) followed by
48 hours at temperature not exceeding
25°C If not used immediately, the in
use storage time and conditions are the
responsibility of the user
PL 00116/0300
PA 167/98/4
Baxter Healthcare Limited
Caxton Way
Thetford
Norfolk
IP24 3SE
United Kingdom
POM Symbol
CLINIMIX N14G30E
Solution for infusion 2000 ml
TH-35-01-780
EXP
2600
2000
1600
1000
800
600
400
200
| CLINIMIX
isoleucine, leucine, valine, lysine, methionine, phenylalanine, threonine, tryptophan, arginine, histidine, alanine, glycine, proline, serine, tyrosine, sodium acetate, dibasic potassium phosphate, sodium chloride, magnesium chloride, dextrose monohydrate, calcium chloride solution |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CLINIMIX
isoleucine, leucine, valine, lysine, methionine, phenylalanine, threonine, tryptophan, arginine, histidine, alanine, glycine, proline, serine, tyrosine, sodium acetate, dibasic potassium phosphate, sodium chloride, magnesium chloride, dextrose monohydrate, calcium chloride solution |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CLINIMIX
isoleucine, leucine, valine, lysine, methionine, phenylalanine, threonine, tryptophan, arginine, histidine, alanine, glycine, proline, serine, tyrosine, sodium acetate, dibasic potassium phosphate, sodium chloride, magnesium chloride, dextrose monohydrate, calcium chloride solution |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Baxter Healthcare Corporation (005083209) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Baxter Healthcare Ltd | 221478644 | ANALYSIS(0338-9566, 0338-9570, 0338-9587) , LABEL(0338-9566, 0338-9570, 0338-9587) , MANUFACTURE(0338-9566, 0338-9570, 0338-9587) , PACK(0338-9566, 0338-9570, 0338-9587) , STERILIZE(0338-9566, 0338-9570, 0338-9587) | |