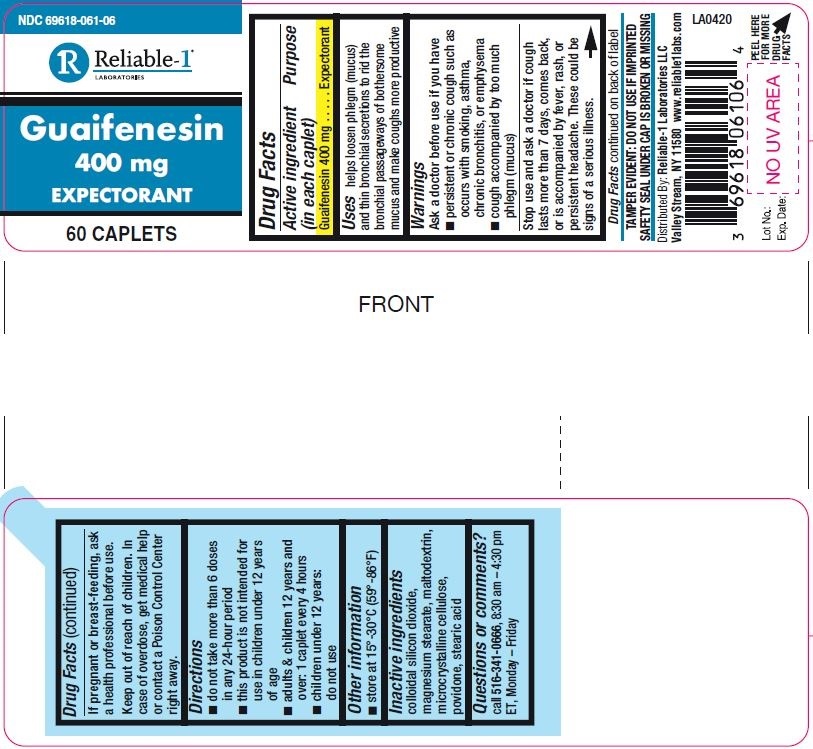
Label: GUAIFENESIN tablet
- NDC Code(s): 69618-061-06
- Packager: Reliable 1 Laboratories LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated March 29, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient (in each caplet)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- cough accompanied by too much phlegm (mucus)
Stop use and ask a doctor if cough lasts more then 7 days, comes back, or is accompanied by fever, rash, or persistent headache. These could be signes of serious illness.
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GUAIFENESIN
guaifenesin tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69618-061 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 400 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POVIDONE (UNII: FZ989GH94E) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) Product Characteristics Color white Score no score Shape OVAL Size 17mm Flavor Imprint Code A;152 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69618-061-06 60 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 08/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 08/01/2020 Labeler - Reliable 1 Laboratories LLC (079718111) Registrant - Reliable 1 Laboratories LLC (079718111)