FLUCELVAX QUADRIVALENT (PRE-FILLED SYRINGE)
- influenza a virus a/washington/19/2020 (h1n1) antigen (mdck cell derived, propiolactone inactivated), influenza a virus a/tasmania/503/2020 (h3n2) antigen (mdck cell derived, propiolactone inactivated), influenza b virus b/darwin/7/2019 antigen (mdck cell derived, propiolactone inactivated), influenza b virus b/singapore/inftt-16-0610/2016 antigen (mdck cell derived, propiolactone inactivated) injection, suspension
FLUCELVAX QUADRIVALENT (MULTI-DOSE VIAL)
- influenza a virus a/washington/19/2020 (h1n1) antigen (mdck cell derived, propiolactone inactivated), influenza a virus a/tasmania/503/2020 (h3n2) antigen (mdck cell derived, propiolactone inactivated), influenza b virus b/darwin/7/2019 antigen (mdck cell derived, propiolactone inactivated), influenza b virus b/singapore/inftt-16-0610/2016 antigen (mdck cell derived, propiolactone inactivated) injection, suspension
Seqirus Inc.
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use FLUCELVAX® QUADRIVALENT safely and effectively. See full prescribing information for FLUCELVAX QUADRIVALENT.
FLUCELVAX QUADRIVALENT (Influenza Vaccine) Suspension for Intramuscular Injection 2021-2022 Formula Initial U.S. Approval: 2016 INDICATIONS AND USAGEDOSAGE AND ADMINISTRATIONFor intramuscular use only (2)
DOSAGE FORMS AND STRENGTHSCONTRAINDICATIONSWARNINGS AND PRECAUTIONSIf Guillain-Barré syndrome has occurred within 6 weeks of receipt of a prior influenza vaccine, the decision to give FLUCELVAX QUADRIVALENT should be based on careful consideration of the potential benefits and risks. (5.1) ADVERSE REACTIONS
To report SUSPECTED ADVERSE REACTIONS, contact Seqirus at 1-855-358-8966 or VAERS at 1-800-822-7967 or www.vaers.hhs.gov. USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION. Revised: 10/2021 |
|||||||||||||
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
FLUCELVAX QUADRIVALENT is an inactivated vaccine indicated for active immunization for the prevention of influenza disease caused by influenza virus subtypes A and types B contained in the vaccine. FLUCELVAX QUADRIVALENT is approved for use in persons 6 months of age and older. [see Clinical Studies (14)]
2 DOSAGE AND ADMINISTRATION
For intramuscular injection only.
2.1 Dosage and Schedule
Administer FLUCELVAX QUADRIVALENT as a single 0.5 mL intramuscular injection.
|
1 1 or 2 doses depends on vaccination history as per Advisory Committee on Immunization Practices annual recommendations on prevention and control of influenza with vaccines. |
||
| Age | Dose | Schedule |
| 6 months through 8 years of age | One or two doses1, 0.5 mL each | If 2 doses, administer at least 4 weeks apart |
| 9 years of age and older | One dose, 0.5 mL | Not Applicable |
2.2 Administration
Shake the syringe vigorously before administering and shake the multi-dose vial preparation each time before withdrawing a dose of vaccine. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit. [see Description (11)] If either condition exists, do not administer the vaccine. Between uses, return the multi-dose vial to the recommended storage conditions between 2º and 8ºC (36º and 46ºF). Do not freeze. Discard if the vaccine has been frozen.
Administer intramuscularly only, preferably in the region of the deltoid muscle of the upper arm. Younger children with insufficient deltoid mass should be vaccinated in the anterolateral aspect of the thigh.
3 DOSAGE FORMS AND STRENGTHS
FLUCELVAX QUADRIVALENT is a suspension for injection supplied in two presentations:
- a 0.5 mL single-dose pre-filled Luer Lock syringe
- a 5 mL multi-dose vial containing 10 doses (each dose is 0.5 mL)
4 CONTRAINDICATIONS
Do not administer FLUCELVAX QUADRIVALENT to anyone with a history of severe allergic reaction (e.g. anaphylaxis) to any component of the vaccine [see Description (11)].
5 WARNINGS AND PRECAUTIONS
5.1 Guillain-Barré Syndrome
The 1976 swine influenza vaccine was associated with an elevated risk of Guillain-Barré syndrome (GBS). Evidence for a causal relation of GBS with other influenza vaccines is inconclusive; if an excess risk exists, it is probably slightly more than 1 additional case per 1 million persons vaccinated.1 If GBS has occurred after receipt of a prior influenza vaccine, the decision to give FLUCELVAX QUADRIVALENT should be based on careful consideration of the potential benefits and risks.
5.2 Preventing and Managing Allergic Reactions
Appropriate medical treatment and supervision must be available to manage possible anaphylactic reactions following administration of the vaccine.
5.3 Syncope
Syncope (fainting) can occur in association with administration of injectable vaccines, including FLUCELVAX QUADRIVALENT. Syncope can be accompanied by transient neurological signs such as visual disturbance, paresthesia, and tonic-clonic limb movements. Procedures should be in place to avoid falling injury and to restore cerebral perfusion following syncope by maintaining a supine or Trendelenburg position.
6 ADVERSE REACTIONS
In children 6 months through 3 years of age who received FLUCELVAX QUADRIVALENT, the most commonly reported injection-site adverse reactions were tenderness (27.9%), erythema (25.8%), induration (17.3%) and ecchymosis (10.7%). The most common systemic adverse reactions were irritability (27.9%), sleepiness (26.9%), diarrhea (17.9%) and change of eating habits (17.4%).
In children 2 through 8 years of age who received FLUCELVAX QUADRIVALENT, the most commonly reported injection-site adverse reactions were tenderness (28.7%), pain (27.9%) and erythema (21.3%), induration (14.9%) and ecchymosis (10.0%). The most common systemic adverse reactions were sleepiness (14.9%), headache (13.8%), fatigue (13.8%), irritability (13.8%) and loss of appetite (10.6%).
In children and adolescents 9 through 17 years of age who received FLUCELVAX QUADRIVALENT, the most commonly reported injection-site adverse reactions were injection site pain (21.7%), erythema (17.2%) and induration (10.5%). The most common systemic adverse reactions were headache (18.1%) and fatigue (17.0%).
In adults 18 through 64 years of age who received FLUCELVAX QUADRIVALENT, the most commonly reported (≥10%) injection-site adverse reactions were pain (45.4%), erythema (13.4%) and induration (11.6%). The most common systemic adverse reactions were headache (18.7%), fatigue (17.8%) and myalgia (15.4%).
In adults ≥65 years of age who received FLUCELVAX QUADRIVALENT, the most commonly reported injection-site adverse reactions were pain (21.6%) and erythema (11.9%).
6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a vaccine cannot be directly compared to rates in clinical studies of another vaccine and may not reflect rates observed in clinical practice.
Children and Adolescents 6 months through 17 years of age:
The safety of FLUCELVAX QUADRIVALENT was evaluated in children and adolescents in two clinical studies: Study 1 and 2.
Study 1 was a randomized, observer-blind, multicenter study in children 6 months through 3 years of age. The safety population included a total of 2402 children 6 months through 3 years of age who received FLUCELVAX QUADRIVALENT (N=1597) or a US-licensed quadrivalent influenza vaccine comparator, AFLURIA QUADRIVALENT (N=805). In the safety population, 894 subjects (37.2%) were 6 months through 23 months of age, and 1508 subjects (62.8%) were 24 months through 47 months of age. The solicited safety set consisted of 2348 subjects who received FLUCELVAX QUADRIVALENT (N=1564) or a US-licensed quadrivalent influenza vaccine comparator (N=784). Study subjects received one or two doses (separated by 4 weeks) of FLUCELVAX QUADRIVALENT or the comparator vaccine depending on the subject's prior influenza vaccination history.
In this study, solicited local injection site and systemic adverse reactions were collected on a symptom diary card for 7 days following vaccination.
In children 6 months through 3 years of age, the incidence of local and systemic solicited adverse reactions reported by children who received FLUCELVAX QUADRIVALENT and comparator are summarized in Table 2.
| Percentage (%)2 of participants Reporting a Reaction | ||||||||
|---|---|---|---|---|---|---|---|---|
| Participants 6 through 23 months | Participants 24 through 47 months | |||||||
| FLUCELVAX QUADRIVALENT N=581 | Comparator3
N=292 | FLUCELVAX QUADRIVALENT N=983 | Comparator3
N=492 |
|||||
| Any | Gr 3 | Any | Gr 3 | Any | Gr 3 | Any | Gr 3 | |
|
Abbreviations: Gr 3, Grade 3. |
||||||||
|
N = number of participants in the Safety Population for each study vaccine group. |
||||||||
|
1 Solicited Safety Population: participants who were vaccinated and provided any solicited local or systemic adverse reaction safety data on subject diary cards from Day 1 through Day 7 after vaccination. |
||||||||
|
2 Proportion of participants reporting each solicited local adverse reaction or systemic adverse event by study vaccine group based on the number of participants contributing any follow up safety information for at least one data value of an individual sign/symptom |
||||||||
|
3 Comparator: US-Licensed Quadrivalent Influenza vaccine |
||||||||
|
4 Local adverse reactions: Grade 3 tenderness defined as, “Cried when limb was moved/spontaneously painful” in subjects 6 through 23 months, and “Prevents daily activity” in subjects 24 months and older; Erythema, induration and ecchymosis: any = ≥1mm diameter, Grade 3 =>50 mm diameter. |
||||||||
|
5 Systemic adverse reactions: Fever: any = ≥100.4°F , Grade 3 = ≥102.2°F (either rectal, oral, axillary, or tympanic membrane); Grade 3 change of eating habits: Missed more than 2 feeds/meals; Grade 3 sleepiness: Sleeps most of the time and is hard to arouse him/her; Grade 3 vomiting: 6 or more times in 24 hours or requires intravenous hydration; Grade 3 diarrhea: 6 or more loose stools in 24 hours or requires intravenous hydration; Grade 3 irritability: unable to console. Grade 3 for all other adverse reactions is that which prevents daily activity. |
||||||||
|
The rates of antipyretic or analgesic use reported on the diary card for prophylaxis or treatment of high temperature or pain were as follows: 6 through 23 months of age FLUCELVAX QUADRIVALENT 20.3%, Comparator 23.6%; 24 through 47 months of age FLUCELVAX QUADRIVALENT 12.4%, Comparator 13.6%. |
||||||||
|
Study 1: NCT 04074928 |
||||||||
| Local Adverse Reactions4 | ||||||||
| Tenderness | 25.5 | 2.1 | 23.3 | 1.4 | 29.3 | 2.2 | 33.9 | 1.4 |
| Erythema | 25.3 | 0 | 18.2 | 0 | 26.0 | 0.7 | 28.5 | 0 |
| Induration | 16.5 | 0.5 | 12.0 | 0 | 17.7 | 0.3 | 18.3 | 0 |
| Ecchymosis | 11.2 | 0.2 | 7.5 | 0 | 10.5 | 0.1 | 12.8 | 0 |
| Systemic Adverse Reactions5 | ||||||||
| Irritability | 35.1 | 5.2 | 35.6 | 2.1 | 23.6 | 1.8 | 26.0 | 3.0 |
| Sleepiness | 35.5 | 2.4 | 30.5 | 1.7 | 21.8 | 1.9 | 22.6 | 1.2 |
| Diarrhea | 23.2 | 2.4 | 20.2 | 0.7 | 14.8 | 1.1 | 14.0 | 1.2 |
| Change of eating habits | 21.0 | 1.7 | 21.9 | 2.4 | 15.3 | 1.4 | 15.0 | 1.2 |
| Fever | 9.3 | 0.7 | 10.3 | 0 | 5.4 | 0.6 | 4.8 | 0.2 |
| Vomiting | 10.5 | 0.7 | 6.8 | 0.7 | 4.6 | 0.5 | 5.9 | 0.4 |
| Shivering | 3.1 | 0.2 | 3.1 | 0 | 3.3 | 0.2 | 3.7 | 0 |
In children who received two doses, the rates of solicited local and systemic adverse reactions were generally similar or lower after the second dose compared to the first dose.
All unsolicited adverse events were collected for 28 days after last vaccination. In children 6 months through 3 years of age, unsolicited adverse events were reported in 26.2% of subjects who received FLUCELVAX QUADRIVALENT and 25.7% of subjects who received the US-licensed quadrivalent influenza vaccine comparator within 28 days after last vaccination.
In children 6 months through 3 years of age, serious adverse events (SAEs) were collected throughout the study duration (until 6 months after last vaccination) and were reported by 0.9% of the subjects who received FLUCELVAX QUADRIVALENT and 0.9% of subjects who received the US-licensed quadrivalent influenza vaccine comparator. None of the SAEs were assessed as being related to study vaccine.
Study 2 was a multi-season, multi-national (Australia, Estonia, Finland, Lithuania, Philippines, Poland, Spain, Thailand), randomized, observer-blind study in children and adolescents 2 through 17 years of age. The solicited safety population included a total of 4509 children and adolescents 2 through 17 years of age who received FLUCELVAX QUADRIVALENT(N=2255) or a non-influenza (meningococcal (Groups A, C, Y, and W-135) oligosaccharide diphtheria CRM197 conjugate) comparator vaccine (N=2254).
Children 2 through 8 years of age received one or two doses (separated by 4 weeks) of FLUCELVAX QUADRIVALENT or comparator vaccine depending on the subject's prior influenza vaccination history. Children in the 2-dose comparator group received non-influenza comparator as the first dose and saline placebo as the second dose. Children and adolescents 9 through 17 years of age received a single dose of FLUCELVAX QUADRIVALENT or non-influenza comparator vaccine.
In this study, solicited local injection site and systemic adverse reactions were collected on a symptom diary card for 7 days following vaccination.
In children 2 through 8 and children and adolescents 9 through 17 years of age, the incidence of local and systemic solicited adverse reactions reported by children and adolescents who received FLUCELVAX QUADRIVALENT and comparator are summarized in Table 3.
| Percentage (%)2 of participants in each Age Cohort Reporting a Reaction | ||||||||
|---|---|---|---|---|---|---|---|---|
| Participants 2 through 8 years | Participants 9 through 17 years | |||||||
| FLUCELVAX QUADRIVALENT N=559-1143 | Comparator3
N=562-1142 | FLUCELVAX QUADRIVALENT N=1096-1109 | Comparator3
N=1100-1108 |
|||||
|
Abbreviations: Gr 3, Grade 3. |
||||||||
|
N = number of participants in the Safety Population for each study vaccine group. |
||||||||
|
1 Solicited Safety Population: participants who were vaccinated and provided any solicited local or systemic adverse reaction safety data on subject diary cards from Day 1 through Day 7 after vaccination. |
||||||||
|
2 Proportion of participants reporting each solicited local adverse reaction or systemic adverse event by study vaccine group based on the number of participants contributing any follow up safety information for at least one data value of an individual sign/symptom |
||||||||
|
3 Non-influenza vaccine comparator: MENVEO, meningococcal (Groups A, C, Y, and W-135) oligosaccharide diphtheria CRM197 conjugate vaccine (GlaxoSmithKline Biologicals SA); children assigned to 2 doses received saline placebo as the second dose. |
||||||||
|
4 Local adverse reactions: Grade 3 pain is that which prevents daily activity; Erythema, induration and ecchymosis: any = ≥ 1mm diameter, Grade 3 = > 50 mm diameter for 2 through 5 years and > 100 mm diameter for 6 through 17 years. |
||||||||
|
5 Tenderness, change in eating habits, sleepiness, and irritability were collected for participants 2 through 6 years of age only. |
||||||||
|
6 Systemic adverse reactions: Fever: any = ≥ 100.4°F (Oral), Grade 3 = ≥ 102.2°F (Oral); Grade 3 change of eating habits: Missed more than 2 feeds/meals; Grade 3 sleepiness: Sleeps most of the time and is hard to arouse him/her; Grade 3 vomiting: 6 or more times in 24 hours or requires intravenous hydration; Grade 3 diarrhea: 6 or more loose stools in 24 hours or requires intravenous hydration; Grade 3 irritability: unable to console. Grade 3 for all other adverse events is that which prevents daily activity. |
||||||||
|
The rates of antipyretic or analgesic use reported on the diary card for prophylaxis or treatment of high temperature or pain were as follows: 2 through 8 years of age FLUCELVAX QUADRIVALENT 11.0%, Comparator 7.7%; 9 through 18 years of age FLUCELVAX QUADRIVALENT 6.7%, Comparator 7.1%. |
||||||||
|
Study 2: NCT03165617 |
||||||||
| Any | Gr 3 | Any | Gr 3 | Any | Gr 3 | Any | Gr 3 | |
| Local Adverse Reactions4 | ||||||||
| Tenderness5 | 28.7 | 1.0 | 25.4 | 1.4 | - | - | - | - |
| Pain | 27.9 | 1.2 | 20.3 | 1.6 | 21.7 | 0.5 | 18.3 | 1.0 |
| Erythema | 21.3 | 0.4 | 23.7 | 1.1 | 17.2 | 0 | 18.7 | 0.5 |
| Induration | 14.9 | 0.2 | 15.2 | 0.4 | 10.5 | 0.1 | 11.0 | 0.2 |
| Ecchymosis | 10.0 | 0 | 7.5 | 0.1 | 5.0 | 0 | 5.2 | 0 |
| Systemic Adverse Reactions6 | ||||||||
| Sleepiness5 | 14.9 | 0.9 | 17.6 | 1.8 | - | - | - | - |
| Headache | 13.8 | 0.4 | 11.8 | 0.5 | 18.1 | 1.4 | 17.4 | 0.6 |
| Fatigue | 13.8 | 0.9 | 12.7 | 0.7 | 17.0 | 1.1 | 18.2 | 1.2 |
| Irritability5 | 13.8 | 0.2 | 10.8 | 0.5 | - | - | - | - |
| Loss of Appetite | 10.6 | 0.5 | 8.0 | 0.5 | 8.5 | 0.5 | 7.5 | 0.5 |
| Change of eating habits5 | 9.9 | 1.0 | 10.1 | 0.7 | - | - | - | - |
| Fever | 7.6 | 0.5 | 6.1 | 0.2 | 2.8 | 0.1 | 3.0 | 0.3 |
| Diarrhea | 6.5 | 0.4 | 6.8 | 0.6 | 7.4 | 0.5 | 8.1 | 0.3 |
| Arthralgia | 5.2 | 0.4 | 6.2 | 0.3 | 7.1 | 0.4 | 8.4 | 0.5 |
| Nausea | 5.2 | 0 | 4.5 | 0.7 | 6.0 | 0.2 | 6.1 | 0.6 |
| Vomiting | 4.9 | 0.6 | 4.1 | 0.6 | 3.0 | 0.3 | 3.0 | 0.4 |
| Shivering/Chills | 4.7 | 0.7 | 3.9 | 0.4 | 7.6 | 0.4 | 7.6 | 0.3 |
| Myalgia | 2.9 | 0.2 | 4.0 | 0.3 | 6.1 | 0.5 | 5.5 | 0.5 |
In children who received a second dose (N=762) of FLUCELVAX QUADRIVALENT, the rates of solicited local and systemic adverse reactions were generally lower after the second dose compared to the first dose.
Serious adverse events (SAEs) were collected throughout the study duration (until 6 months after last vaccination) and were reported by 1.1% of the children and adolescents who received FLUCELVAX QUADRIVALENT. None of the SAEs were assessed as being related to study vaccine.
Adults 18 years of age and older:
The safety of FLUCELVAX QUADRIVALENT in adults was evaluated in a randomized, double-blind, controlled study conducted in the US (Study 3). The safety population included a total of 2680 adults 18 years of age and older; 1340 adults 18 through 64 years of age and 1340 adults 65 years of age and older.
In this study, adults received FLUCELVAX QUADRIVALENT or one of the two formulations of comparator trivalent influenza vaccine (TIV1c and TIV2c) (FLUCELVAX QUADRIVALENT (N=1335), TIV1c, N=676 or TIV2c, N= 669). The mean age of adults who received FLUCELVAX QUADRIVALENT was 57.4 years of age; 54.8% of adults were female and 75.6% were Caucasian, 13.4% were Black, 9.1% were Hispanics, 0.7% were American Indian and 0.3%, 0.1% and 0.7% were Asian, Native Hawaiian and others, respectively. The safety data observed are summarized in Table 2.
In this study, solicited local injection site and systemic adverse reactions were collected from adults who completed a symptom diary card for 7 days following vaccination.
Solicited adverse reactions for FLUCELVAX QUADRIVALENT and comparator are summarized in Table 4.
| Percentage (%)2 of participants in each Age Cohort Reporting a Reaction | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Participants 18 through 64 years | Participants ≥ 65 years | |||||||||||
| FLUCELVAX Quadrivalent N=663 | TIV1c N=330 | TIV2c N=327 | FLUCELVAX Quadrivalent N=656 | TIV1c N=340 | TIV2c N=336 |
|||||||
|
Abbreviations: Gr 3, Grade 3. |
||||||||||||
|
N = number of participants in the Safety Population for each study vaccine group. |
||||||||||||
|
1 Safety population: all participants in the exposed population who provided post-vaccination safety data |
||||||||||||
|
2 Proportion of participants reporting each solicited local adverse reaction or systemic adverse reaction by study vaccine group based on the number of participants contributing any follow up safety information for at least one data value of an individual sign/symptom |
||||||||||||
|
3 Local Adverse reactions: Grade 3 pain is that which prevents daily activity; Erythema, induration and ecchymosis: any = ≥ 1mm diameter, Grade 3 = > 100 mm diameter. |
||||||||||||
|
4 Systemic adverse reactions: Fever: any = ≥ 100.4°F (Oral), Grade 3 = ≥ 102.2°F (Oral); Grade 3 vomiting: requires outpatient hydration; Grade 3 diarrhea: 6 or more stools or requires outpatient IV hydration; Grade 3 for all other adverse reactions is that which prevents daily activity. |
||||||||||||
|
Study 3: NCT01992094 |
||||||||||||
| Any | Gr 3 | Any | Gr 3 | Any | Gr 3 | Any | Gr 3 | Any | Gr 3 | Any | Gr 3 | |
| Local Adverse Reactions3 | ||||||||||||
| Pain | 45.4 | 0.5 | 37.0 | 0.3 | 40.7 | 0 | 21.6 | 0 | 18.8 | 0 | 18.5 | 0 |
| Erythema | 13.4 | 0 | 13.3 | 0 | 10.1 | 0 | 11.9 | 0 | 10.6 | 0 | 10.4 | 0 |
| Induration | 11.6 | 0 | 9.7 | 0.3 | 10.4 | 0 | 8.7 | 0 | 6.8 | 0 | 7.7 | 0 |
| Ecchymosis | 3.8 | 0 | 3.3 | 0.3 | 5.2 | 0 | 4.7 | 0 | 4.4 | 0 | 5.4 | 0 |
| Systemic Adverse Reactions4 | ||||||||||||
| Headache | 18.7 | 0.9 | 18.5 | 0.9 | 18.7 | 0.6 | 9.3 | 0.3 | 8.5 | 0.6 | 8.3 | 0.6 |
| Fatigue | 17.8 | 0.6 | 22.1 | 0.3 | 15.6 | 1.5 | 9.1 | 0.8 | 10.6 | 0.3 | 8.9 | 0.6 |
| Myalgia | 15.4 | 0.8 | 14.5 | 0.9 | 15.0 | 1.2 | 8.2 | 0.2 | 9.4 | 0.3 | 8.3 | 0.6 |
| Nausea | 9.7 | 0.3 | 7.3 | 0.9 | 8.9 | 1.2 | 3.8 | 0.2 | 4.1 | 0 | 4.2 | 0.3 |
| Loss of appetite | 8.3 | 0.3 | 8.5 | 0.3 | 8.3 | 0.9 | 4.0 | 0.2 | 5.0 | 0 | 3.6 | 0.3 |
| Arthralgia | 8.1 | 0.5 | 8.2 | 0 | 9.5 | 0.9 | 5.5 | 0.5 | 5.0 | 0.3 | 6.8 | 0.9 |
| Diarrhea | 7.4 | 0.6 | 7.6 | 0 | 7.6 | 0.6 | 4.3 | 0.5 | 5.0 | 0.9 | 5.1 | 0.3 |
| Chills | 6.2 | 0.2 | 6.4 | 0.6 | 6.4 | 0 | 4.4 | 0.3 | 4.1 | 0.3 | 4.5 | 0.6 |
| Vomiting | 2.6 | 0 | 1.5 | 0.3 | 0.9 | 0 | 0.9 | 0.2 | 0.3 | 0 | 0.6 | 0 |
| Fever | 0.8 | 0 | 0.6 | 0 | 0.3 | 0 | 0.3 | 0 | 0.9 | 0 | 0.6 | 0 |
Unsolicited adverse events were collected for 21 days after vaccination. In adults 18 years of age and older, unsolicited adverse events were reported in 16.1% of adults who received FLUCELVAX QUADRIVALENT, within 21 days after vaccination.
In adults 18 years of age and older, serious adverse events (SAEs) were collected throughout the study duration (until 6 months after vaccination) and were reported by 3.9%, of the adults who received FLUCELVAX QUADRIVALENT. None of the SAEs were assessed as being related to study vaccine.
6.2 Postmarketing Experience
The following additional adverse events have been identified during post-approval use of FLUCELVAX QUADRIVALENT. Because these events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to the vaccine.
Immune system disorders: Allergic or immediate hypersensitivity reactions, including anaphylactic shock.
Nervous systems disorders: Syncope, presyncope, paresthesia.
Skin and subcutaneous tissue disorders: Generalized skin reactions including pruritus, urticaria or non-specific rash.
General disorders and administration site conditions: Extensive swelling of injected limb.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
All pregnancies have a risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively. There are insufficient data for FLUCELVAX QUADRIVALENT in pregnant women to inform vaccine-associated risks in pregnancy.
There were no developmental toxicity studies of FLUCELVAX QUADRIVALENT performed in animals. A developmental toxicity study has been performed in female rabbits administered FLUCELVAX (trivalent formulation) prior to mating and during gestation. The dose was 0.5 mL on each occasion (a single human dose is 0.5 mL). This study revealed no evidence of harm to the fetus due to FLUCELVAX (trivalent formulation).
Data
Animal Data
In a developmental toxicity study, female rabbits were administered FLUCELVAX (trivalent formulation) by intramuscular injection 1, 3, and 5 weeks prior to mating, and on gestation days 7 and 20. The dose was 0.5 mL on each occasion (a single human dose is 0.5 mL). No vaccine-related fetal malformations or variations and no adverse effects on pre-weaning development were observed in the study.
8.2 Lactation
Risk Summary
It is not known whether FLUCELVAX QUADRIVALENT is excreted in human milk. Data are not available to assess the effects of FLUCELVAX QUADRIVALENT on the breastfed infant or on milk production/excretion.
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for FLUCELVAX QUADRIVALENT and any potential adverse effects on the breastfed child from FLUCELVAX QUADRIVALENT or from the underlying maternal condition. For preventive vaccines, the underlying maternal condition is susceptibility to disease prevented by the vaccine or the effects on milk production.
8.4 Pediatric Use
Safety and effectiveness have not been established in children less than 6 months of age.
8.5 Geriatric Use
Of the total number of adults who received one dose of FLUCELVAX QUADRIVALENT in clinical studies and included in the safety population (2493), 26% (660) were 65 years of age and older and 8% (194) were 75 years of age or older.
Antibody responses to FLUCELVAX QUADRIVALENT were lower in the geriatric (adults 65 years and older) population than in younger adults. [see Clinical Studies (14.3)]
11 DESCRIPTION
FLUCELVAX QUADRIVALENT (Influenza Vaccine) is a subunit influenza vaccine manufactured using cell derived candidate vaccine viruses (CVV) that are propagated in Madin Darby Canine Kidney (MDCK) cells, a continuous cell line. These cells were adapted to grow freely in suspension in culture medium. The virus is inactivated with β-propiolactone, disrupted by the detergent cetyltrimethylammonium bromide and purified through several process steps. Each of the 4 virus strains is produced and purified separately then pooled to formulate the quadrivalent vaccine.
FLUCELVAX QUADRIVALENT is a sterile, slightly opalescent suspension in phosphate buffered saline. FLUCELVAX QUADRIVALENT is standardized according to United States Public Health Service requirements for the 2021-2022 influenza season and is formulated to contain a total of 60 micrograms (mcg) hemagglutinin (HA) per 0.5 mL dose in the recommended ratio of 15 mcg HA of each of the following four influenza strains:
A/Washington/19/2020 (an A/Wisconsin/588/2019 (H1N1)pdm09-like virus);
A/Tasmania/503/2020 (an A/Cambodia/e0826360/2020 (H3N2)-like virus);
B/Darwin/7/2019 (a B/Washington/02/2019-like virus);
B/Singapore/INFTT-16-0610/2016 (a B/Phuket/3073/2013-like virus).
Each dose of FLUCELVAX QUADRIVALENT may contain residual amounts of MDCK cell protein (≤ 25.2 mcg), protein other than HA (≤ 240 mcg), MDCK cell DNA (≤ 10 ng), polysorbate 80 (≤ 1500 mcg), cetyltrimethylammonium bromide (≤ 18 mcg), and β-propiolactone (≤ 0.5 mcg), which are used in the manufacturing process.
FLUCELVAX QUADRIVALENT contains no egg protein or antibiotics.
FLUCELVAX QUADRIVALENT 0.5 mL pre-filled syringes contain no preservative.
FLUCELVAX QUADRIVALENT 5 mL multi-dose vials contain thimerosal, a mercury derivative, added as a preservative. Each 0.5 mL dose from the multi-dose vial contains 25 mcg mercury.
The tip caps and plungers of the pre-filled syringes and the multi-dose vial stopper are not made with natural rubber latex.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Influenza illness and its complications follow infection with influenza viruses. Global surveillance and analysis of influenza virus isolates permits identification of yearly antigenic variants. Since 1977, antigenic variants of influenza A (H1N1 and H3N2) viruses and influenza B viruses have been in global circulation. Specific levels of hemagglutination inhibition (HI) antibody titers induced by vaccination with inactivated influenza virus vaccine have not been correlated with protection from influenza illness. In some studies, HI antibody titers of ≥1:40 have been associated with protection from influenza illness in up to 50% of adults.2,3
Antibody against one influenza virus type or subtype confers little or no protection against another. Furthermore, antibody to one antigenic variant of influenza virus might not protect against a new antigenic variant of the same type or subtype. Frequent development of antigenic variants through antigenic drift is the virologic basis for seasonal epidemics and the reason for the usual change of one or more strains in each year's influenza vaccine. Therefore, inactivated influenza vaccines are standardized to contain the hemagglutinin of influenza virus strains representing the influenza viruses likely to circulate in the United States in the upcoming winter.
Annual influenza vaccination is recommended by the Advisory Committee on Immunization Practices because immunity declines during the year after vaccination, and because circulating strains of influenza virus change from year to year.4
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
FLUCELVAX QUADRIVALENT has not been evaluated for carcinogenic or mutagenic potential, or for impairment of male fertility in animals.
FLUCELVAX (trivalent formulation) administered to female rabbits had no effect on fertility. [see Use in Specific Population (8.1)]
14 CLINICAL STUDIES
14.1 Efficacy against Culture-Confirmed Influenza
The efficacy experience with FLUCELVAX is relevant to FLUCELVAX QUADRIVALENT because both vaccines are manufactured using the same process and have overlapping compositions.
A multinational (US, Finland, and Poland), randomized, observer-blind, placebo-controlled trial was performed to assess clinical efficacy and safety of FLUCELVAX during the 2007-2008 influenza season in adults aged 18 through 49 years (Study 4). A total of 11,404 adults were enrolled to receive FLUCELVAX (N=3828), AGRIFLU (N=3676) or placebo (N=3900) in a 1:1:1 ratio. Among the overall study population enrolled, the mean age was 33 years, 55% were female, 84% were Caucasian, 7% were Black, 7% were Hispanic, and 2% were of other ethnic origin.
FLUCELVAX efficacy was assessed by the prevention of culture-confirmed symptomatic influenza illness caused by viruses antigenically matched to those in the vaccine and prevention of influenza illness caused by all influenza viruses compared to placebo. Influenza cases were identified by active and passive surveillance of influenza-like illness (ILI). ILI was defined as a fever (oral temperature ≥ 100.0°F / 38°C) and cough or sore throat. Nose and throat swab samples were collected for analysis within 120 hours of onset of an influenza-like illness in the period from 21 days to 6 months after vaccination. Overall vaccine efficacy against all influenza viral subtypes and vaccine efficacy against individual influenza viral subtypes were calculated (Tables 5 and 6, respectively).
|
1Efficacy against influenza was evaluated over a 9-month period in 2007/2008 |
|||||
|
2Simultaneous one-sided 97.5% confidence intervals for the vaccine efficacy (VE) of FLUCELVAX relative to placebo based on the Sidak-corrected score confidence intervals for the relative risk. Vaccine Efficacy = (1 - Relative Risk) x 100 % |
|||||
|
3VE success criterion: the lower limit of the one-sided 97.5% CI for the estimate of the VE relative to placebo is >40% |
|||||
|
Study 4: NCT00630331 |
|||||
| Number of participants per protocol | Number of participants with influenza | Attack Rate
(%) | Vaccine Efficacy (VE)1,2 | ||
| % | Lower Limit of One-
Sided 97.5% CI of VE2, 3 |
||||
| Antigenically Matched Strains | |||||
| FLUCELVAX | 3776 | 7 | 0.19 | 83.8 | 61.0 |
| Placebo | 3843 | 44 | 1.14 | -- | -- |
| All Culture-Confirmed Influenza | |||||
| FLUCELVAX | 3776 | 42 | 1.11 | 69.5 | 55.0 |
| Placebo | 3843 | 140 | 3.64 | -- | -- |
|
1No VE success criterion was prespecified in the protocol for each individual influenza virus subtype. |
||||||
|
2 Simultaneous one-sided 97.5% confidence intervals for the vaccine efficacy (VE) of FLUCELVAX relative to placebo based on the Sidak-corrected score confidence intervals for the relative risk. Vaccine Efficacy = (1 - Relative Risk) x 100 %; |
||||||
|
3 There were too few cases of influenza due to vaccine-matched influenza A/H3N2 or B to adequately assess vaccine efficacy. |
||||||
|
Study 4: NCT00630331 |
||||||
| FLUCELVAX
(N=3776) | Placebo
(N=3843) | Vaccine Efficacy (VE)2 | ||||
| Attack Rate
(%) | Number of Participants with Influenza | Attack Rate
(%) | Number of Participants with Influenza | % | Lower Limit of One-Sided 97.5% CI of VE1,2 | |
| Antigenically Matched Strains | ||||||
| A/H3N23 | 0. 05 | 2 | 0 | 0 | -- | -- |
| A/H1N1 | 0.13 | 5 | 1.12 | 43 | 88.2 | 67.4 |
| B3 | 0 | 0 | 0.03 | 1 | -- | -- |
| All Culture-Confirmed Influenza | ||||||
| A/H3N2 | 0.16 | 6 | 0.65 | 25 | 75.6 | 35.1 |
| A/H1N1 | 0.16 | 6 | 1.48 | 57 | 89.3 | 73.0 |
| B | 0.79 | 30 | 1.59 | 61 | 49.9 | 18.2 |
14.2 Efficacy of FLUCELVAX QUADRIVALENT in Children and Adolescents 2 through 17 Years of Age
Absolute efficacy of FLUCELVAX QUADRIVALENT was evaluated in children and adolescents 2 through 17 years of age in Study 2. This was a multinational, randomized, non-influenza vaccine comparator-controlled efficacy, immunogenicity and safety study conducted in 8 countries during the following 3 influenza seasons: Southern Hemisphere 2017, Northern Hemisphere 2017/2018 and Northern Hemisphere 2018/2019. The study enrolled 4514 children and adolescents. Out of the 4514 enrolled, 4513 received either FLUCELVAX QUADRIVALENT (N=2258) or a non-influenza (meningococcal (Groups A, C, Y, and W-135) oligosaccharide diphtheria CRM197 conjugate) comparator vaccine (N=2255). The full analysis set (FAS) for efficacy consisted of 4509 children and adolescents.
Children 2 through 8 years of age received either one or two doses (separated by 4 weeks) of FLUCELVAX QUADRIVALENT or comparator vaccine depending on the subject's prior influenza vaccination history. Children in the 2-dose comparator group received non-influenza comparator as the first dose and saline placebo as the second dose. Children and adolescents 9 through 17 years of age received a single dose of FLUCELVAX QUADRIVALENT or non-influenza comparator vaccine. Among all enrolled children and adolescents (N=4514), the mean age was 8.8 years, 48% were female, 51% were 2 through 8 years of age, 50% were Caucasian and 49% were Asian. There were no notable differences in the distribution of demographic and baseline characteristics between the two treatment groups.
FLUCELVAX QUADRIVALENT efficacy was assessed by the prevention of confirmed influenza illness caused by any influenza Type A or B strain. Influenza cases were identified by active and passive surveillance of influenza-like illness (ILI) and confirmed by cell culture and/or real-time polymerase chain reaction (RT-PCR). ILI was defined as a fever (oral temperature ≥ 100.0°F / 37.8°C) along with any of the following: cough, sore throat, nasal congestion, or rhinorrhea. The overall vaccine efficacy for the entire study population (2 through 17 years) was 54.6% (95% CI 45.7 – 62.1), which met predefined success criteria. In addition, vaccine efficacy was 50.5% (95% CI 38.4 – 60.2) in children 2 through 8 years of age and 61.9% (95% CI 47.4 – 72.3) in those 9 through 17 years of age. Vaccine efficacy against all influenza viral subtypes and against individual influenza viral subtypes antigenically similar to the subtypes in the vaccine were calculated (Table 7).
| Number of participants per protocol1 | Number of cases of influenza | Attack Rate
(%) | Vaccine Efficacy (VE)2 | ||
|---|---|---|---|---|---|
| VE % | 95% Confidence Interval3 | ||||
|
1Number of participants in the Full-Analysis Set (FAS) – Efficacy, which included all participants randomized, received a study vaccination and provided efficacy data |
|||||
|
2Efficacy against influenza was evaluated over three influenza seasons, SH 2017, NH 2017-18 and NH 2018-19 |
|||||
|
3FLUCELVAX QUADRIVALENT met the pre-defined success criterion defined as the lower limit of the two-sided 95% CI of absolute vaccine efficacy greater than 20% |
|||||
|
4Non-Influenza Comparator: (MENVEO, meningococcal (Groups A, C, Y, and W-135) oligosaccharide diphtheria CRM197 conjugate vaccine, GlaxoSmithKline Biologicals SA); children assigned to 2 doses received saline placebo as the second dose. |
|||||
|
Study 2: NCT03165617 |
|||||
| RT-PCR or Culture Confirmed Influenza | |||||
| FLUCELVAX QUADRIVALENT | 2257 | 175 | 7.8 | 54.6 | 45.7 - 62.1 |
| Non-Influenza Comparator4 | 2252 | 364 | 16.2 | - | - |
| Culture Confirmed Influenza | |||||
| FLUCELVAX QUADRIVALENT | 2257 | 115 | 5.1 | 60.8 | 51.3 - 68.5 |
| Non-Influenza Comparator4 | 2252 | 279 | 12.4 | - | - |
| Antigenically Matched Culture-Confirmed Influenza | |||||
| FLUCELVAX QUADRIVALENT | 2257 | 90 | 4.0 | 63.6 | 53.6 - 71.5 |
| Non-Influenza Comparator4 | 2252 | 236 | 10.5 | - | - |
14.3 Immunogenicity of FLUCELVAX QUADRIVALENT in Adults 18 years of age and above
Immunogenicity of FLUCELVAX QUADRIVALENT was evaluated in adults 18 years of age and older in a randomized, double-blind, controlled study conducted in the US (Study 3). In this study, adults received FLUCELVAX QUADRIVALENT or one of the two formulations of comparator trivalent influenza vaccine (FLUCELVAX QUADRIVALENT (N=1334), TIV1c, N=677 or TIV2c, N=669). In the per protocol set, the mean age of adults who received FLUCELVAX QUADRIVALENT was 57.5 years; 55.1% of adults were female and 76.1% of adults were Caucasian, 13% were black and 9% were Hispanics. The immune response to each of the vaccine antigens was assessed, 21 days after vaccination.
The immunogenicity endpoints were geometric mean antibody titers (GMTs) of hemagglutination inhibition (HI) antibodies response and percentage of adults who achieved seroconversions, defined as a pre-vaccination HI titer of < 1:10 with a post-vaccination titer ≥ 1:40 or a pre-vaccination HI titer > 1:10 and at least 4-fold increase in serum HI antibody titer.
FLUCELVAX QUADRIVALENT was noninferior to TIVc. Noninferiority was established for all 4 influenza strains included in FLUCELVAX QUADRIVALENT, as assessed by ratios of GMTs and the differences in the percentages of adults achieving seroconversion at 3 weeks following vaccination. The antibody response to influenza B strains contained in FLUCELVAX QUADRIVALENT was superior to the antibody response after vaccination with TIVc containing an influenza B strain from the alternate lineage. There was no evidence that the addition of the second influenza B strain resulted in immune interference to other strains included in the vaccine. (See Table 8)
|
Abbreviations: HI = hemagglutination inhibition. PPS = per protocol set. GMT = geometric mean titer. CI = confidence interval. |
|||||
|
1 Per protocol set: All participants in Full Analysis Set, immunogenicity population, who has correctly received the assigned vaccine, have no major protocol deviations leading to exclusion as defined prior to unblinding/ analysis and are not excluded due to other reasons defined prior to unblinding or analysis. |
|||||
|
2 The comparator vaccine for noninferiority comparisons for A/H1N1, A/H3N2 and B1 is TIV1c, for B2 it is TIV2c. |
|||||
|
3 Seroconversion rate = percentage of participants with either a pre-vaccination HI titer < 1:10 and post-vaccination HI titer ≥ 1:40 or with a pre-vaccination HI titer ≥ 1:10 and a minimum 4-fold increase in post-vaccination HI antibody titer |
|||||
|
Study 3: NCT01992094 |
|||||
| FLUCELVAX Quadrivalent N = 1250 | TIV1c/TIV2c2
N = 635/N =639 | Vaccine Group Ratio (95% CI) | Vaccine Group Difference (95% CI) |
||
| A/H1N1 | GMT (95% CI) | 302.8 (281.8-325.5) | 298.9 (270.3-330.5) | 1.0 (0.9-1.1) | - |
| Seroconversion Rate3
(95% CI) | 49.2% (46.4-52.0) | 48.7% (44.7-52.6) | - | -0.5% (-5.3-4.2) |
|
| A/H3N2 | GMT (95% CI) | 372.3 (349.2-396.9) | 378.4 (345.1-414.8) | 1.0 (0.9-1.1) | - |
| Seroconversion Rate3
(95% CI) | 38.3% (35.6-41.1) | 35.6% (31.9-39.5) | - | -2.7% (-7.2-1.9) |
|
| B1 | GMT (95% CI) | 133.2 (125.3-141.7) | 115.6 (106.4-125.6) | 0.9 (0.8-1.0) | - |
| Seroconversion Rate3
(95% CI) | 36.6% (33.9-39.3) | 34.8% (31.1-38.7) | - | -1.8% (-6.2-2.8) |
|
| B2 | GMT (95% CI) | 177.2 (167.6-187.5) | 164.0 (151.4-177.7) | 0.9 (0.9-1.0) | - |
| Seroconversion Rate3 (95% CI) | 39.8% (37.0-42.5) | 35.4% (31.7-39.2) | - | -4.4% (-8.9-0.2) |
|
14.4 Immunogenicity in Children and Adolescents 6 months through 17 years of age
Immunogenicity of FLUCELVAX QUADRIVALENT was evaluated in two clinical studies in children 6 months through 3 years of age (Study 1) and 4 through 17 years of age (Study 5).
Study 1 was a randomized, observer-blind, multicenter study in children 6 months through 3 years of age conducted in the US. In this study, subjects received FLUCELVAX QUADRIVALENT or a US-licensed comparator quadrivalent influenza vaccine (FLUCELVAX QUADRIVALENT N=1597, Comparator QUADRIVALENT (QIV) N=805). In the per protocol set, the mean age of subjects who received FLUCELVAX QUADRIVALENT was 29 months; 49% of subjects were female and 67% of subjects were Caucasian, 27% were Black and <1% were Asian, Hawaiian or other Pacific Islander and American Indian or Alaska Native. Twenty six percent of subjects were of Hispanic origin. The immune response to each of the vaccine antigens was assessed 28 days after last vaccination.
The immunogenicity endpoints were geometric mean antibody titers (GMTs) and percentage of subjects who achieved seroconversion, defined as a pre-vaccination HI or microneutralization (MN) titer of <1:10 with a post-vaccination titer ≥1:40 or with a pre-vaccination HI or MN titer ≥ 1:10 and a minimum 4-fold increase in serum antibody titer. GMTs and seroconversion rates were measured by hemagglutination inhibition (HI) assay for A/H1N1, B/Yamagata and B/Victoria strains and by microneutralization (MN) assay for the A/H3N2 strain.
FLUCELVAX QUADRIVALENT was noninferior to the Comparator QIV. Noninferiority was established for all 4 influenza strains as assessed by ratios of GMTs and the differences in the percentages of subjects achieving seroconversion at 4 weeks following vaccination.
The noninferiority data observed are summarized in Table 9.
|
Abbreviations: GMT = geometric mean titer. CI = confidence interval. |
|||||
|
Assays: GMTs and seroconversion rates were measured by hemagglutination inhibition (HI)* assay for A/H1N1, B/Yamagata and B/Victoria strains and by microneutralization (MN)# assay for the A/H3N2 strain, using cell-derived target viruses. The MN assay was used for A/H3N2 as circulating strains indicated a reduced ability to agglutinate red blood cells. FLUCELVAX QUADRIVALENT was noninferior to the Comparator QIV irrespective of the assay used. HI assay data for A/H3N2: GMT (95%CI) for FLUCELVAX QUADRIVALENT (N=1089) = 288.1 (261.46, 317.54), Comparator QIV (N=575) = 227.6 (201.87, 256.58), Vaccine group ratio (95%CI) = 0.79 (0.69, 0.90), Seroconversion rate (95%CI) for FLUCELVAX QUADRIVALENT (N=1089) = 72.27% (69.51,74.91), Comparator QIV (N=575) = 64.52% (60.46, 68.44), Vaccine Group Difference (95%CI) = -7.75% (-12.51, -3.06). |
|||||
|
Success criteria: The upper bound of the two-sided 95% confidence interval (CI) on the ratio of the GMTs (calculated as GMT US-licensed comparator QIV divided by GMT FLUCELVAX QUADRIVALENT) does not exceed 1.5. The upper bound of the two-sided 95% CI on the difference between the seroconversion rates (calculated as Seroconversion rate US-licensed comparator QIV minus Seroconversion rate FLUCELVAX QUADRIVALENT) does not exceed 10%. |
|||||
|
1 Analyses are performed on data for Day 29 for previously vaccinated subjects and Day 57 for not previously vaccinated subjects |
|||||
|
2 Per protocol set: All participants in Full Analysis Set, immunogenicity population, who have correctly received the assigned vaccine, have no major protocol deviations leading to exclusion as defined prior to unblinding/ analysis and are not excluded due to other reasons defined prior to unblinding or analysis. |
|||||
|
3 Seroconversion rate = percentage of subjects with either a pre-vaccination titer <1:10 and post-vaccination titer ≥1:40 or with a pre-vaccination titer ≥1:10 and a minimum 4-fold increase in post-vaccination antibody titer |
|||||
|
Study 1: NCT 04074928 |
|||||
| FLUCELVAX
QUADRIVALENT | Comparator QIV | Vaccine Group Ratio | Vaccine Group Difference | ||
| A/H1N1* | N = 1092 | N =575 | |||
| GMT (95% CI) | 78.0 (70.75, 86.03) | 57.3 (50.76, 64.63) | 0.73 (0.65, 0.84) | - | |
| Seroconversion Rate 3 (95% CI) | 58.24% (55.25, 61.19) | 46.78% (42.64, 50.96) | - | -11.46 (-16.45, -6.42) |
|
| A/H3N2# | N = 1078 | N = 572 | |||
| GMT (95% CI) | 23.1 (21.21, 25.12) | 23.9 (21.57, 26.57) | 1.04 (0.93, 1.16) | - | |
| Seroconversion Rate 3 (95% CI) | 27.64% (24.99, 30.42) | 30.77% (27.01, 34.73) | - | 3.13 (-1.44, 7.81) |
|
| B/Yamagata* | N = 1092 | N = 575 | |||
| GMT (95% CI) | 35.6 (32.93, 38.58) | 26.0 (23.54, 28.63) | 0.73 (0.66, 0.81) | - | |
| Seroconversion Rate 3 (95% CI) | 46.52% (43.53, 49.53) | 31.65% (27.87, 35.63) | - | -14.87 (-19.61, -9.98) |
|
| B/Victoria* | N = 1092 | N = 575 | |||
| GMT (95% CI) | 22.4 (20.70, 24.19) | 19.6 (17.81, 21.58) | 0.88 (0.79, 0.97) | - | |
| Seroconversion Rate 3 (95% CI) | 30.31% (27.60, 33.13) | 24.35% (20.89, 28.07) | - | -5.96 (-10.33, -1.44) |
|
Study 5 was a randomized, double-blind, controlled study in children and adolescents 4 through 17 years of age conducted in the US. In this study, 1159 children and adolescents received FLUCELVAX QUADRIVALENT. In the per protocol set, the mean age of children and adolescents who received FLUCELVAX QUADRIVALENT was 9.8 years; 47% of children and adolescents were female and 54% of children and adolescents were Caucasian, 22% were black and 19% were Hispanics. The immune response to each of the vaccine antigens was assessed, 21 days after vaccination.
The immunogenicity endpoints were the percentage of children and adolescents who achieved seroconversion, defined as a pre-vaccination hemagglutination inhibition (HI) titer of < 1:10 with a post-vaccination HI titer ≥ 1:40 or at least a 4-fold increase in serum HI titer; and percentage of children and adolescents with a post-vaccination HI titer ≥ 1:40.
In children and adolescents receiving FLUCELVAX QUADRIVALENT, for all four influenza strains, the 95% LBCI seroconversion rates were ≥ 40% and the percentage of children and adolescents who achieved HI titer ≥1:40 post vaccination were ≥ 70% (95% LBCI). (See Table 10)
|
Abbreviations: HI = hemagglutinin inhibition. CI = confidence interval. |
||||
|
Analyses are performed on data for day 22 for previously vaccinated participants and day 50 for not previously vaccinated participants. |
||||
|
1 Seroconversion rate = percentage of participants with either a pre-vaccination HI titer < 1:10 and post-vaccination HI titer ≥ 1:40 or with a pre-vaccination HI titer ≥ 1:10 and a minimum 4-fold increase in post-vaccination HI titer. Immunogenicity success criteria were met if the lower limit of the 95% confidence interval (CI) of the percentage of participants with HI titer ≥ 1:40 is ≥ 70%; and the lower limit of the 95% CI of the percentage of participants with seroconversion is ≥ 40%. |
||||
|
2 Per protocol set: All participants in Full Analysis Set, immunogenicity population, who has correctly received the assigned vaccine, have no major protocol deviations leading to exclusion as defined prior to unblinding/ analysis and are not excluded due to other reasons defined prior to unblinding or analysis. |
||||
|
Study 5: NCT 01992107 |
||||
| A/H1N1 N = 1014 | A/H3N2 N = 1013 | B1 N = 1013 | B2 N = 1009 |
|
| Seroconversion Rate1 (95% CI) | 72% (69-75) | 47% (44-50) | 66% (63-69) | 73% (70-76) |
| HI titer ≥1:40 | 99% (98-100) | 100% (99-100) | 93% (91-94) | 92% (90-93) |
15 REFERENCES
- Lasky T, Terracciano GJ, Magder L, et al. The Guillain-Barré syndrome and the 1992-1993 and 1993-1994 influenza vaccines. N Engl J Med 1998; 339(25):1797-1802.
- Hannoun C, Megas F, Piercy J. Immunogenicity and protective efficacy of influenza vaccination. Virus Res 2004; 103:133-138.
- Hobson D, Curry RL, Beare A, et al. The role of serum hemagglutinin-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg Camb 1972; 767-777.
- Centers for Disease Control and Prevention. Prevention and Control of Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2011; 60(33): 1128-1132
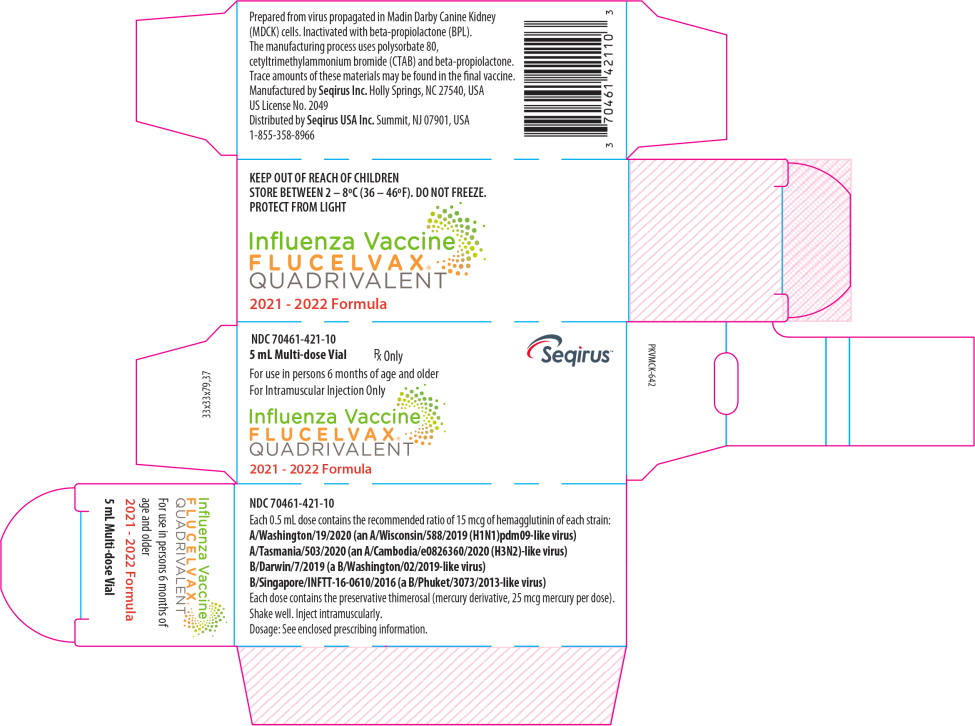
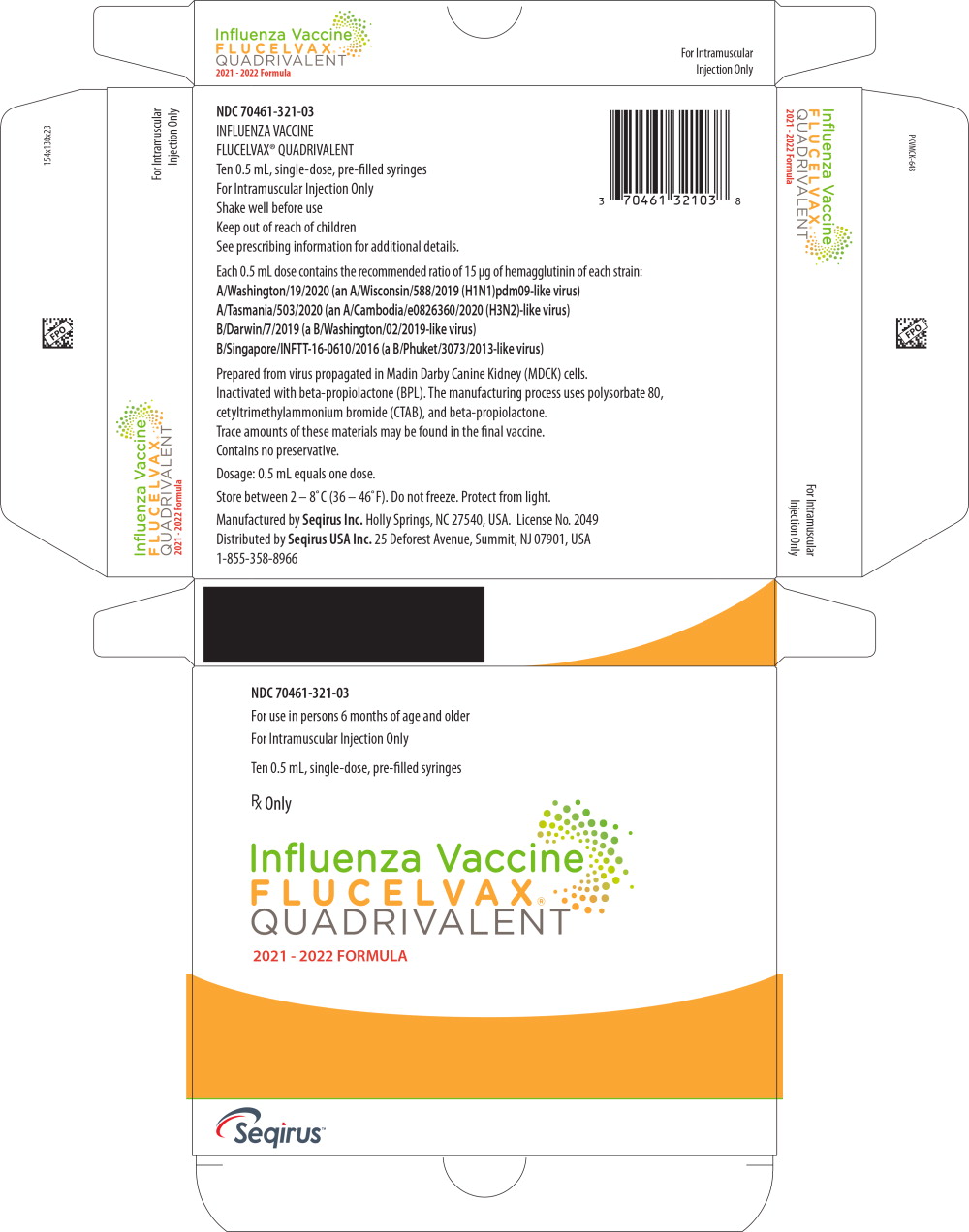
16 HOW SUPPLIED/STORAGE AND HANDLING
FLUCELVAX QUADRIVALENT product presentations are listed in Table 11 below:
| Presentation | Carton NDC Number | Components |
| Pre-filled Syringe | 70461-321-03 | 0.5 mL single dose pre-filled syringe, package of 10 syringes per carton [NDC 70461-321-04] |
| Multi-dose Vial | 70461-421-10 | 5 mL multi-dose vial, individually packaged in a carton [NDC 70461-421-11] |
Store this product refrigerated at 2°C to 8°C (36ºF to 46ºF). Between uses, return the multi-dose vial to the recommended storage conditions. Do not freeze. Protect from light. Do not use after the expiration date.
17 PATIENT COUNSELING INFORMATION
Inform vaccine recipients of the potential benefits and risks of immunization with FLUCELVAX QUADRIVALENT.
Educate vaccine recipients regarding the potential side effects; clinicians should emphasize that (1) FLUCELVAX QUADRIVALENT contains non-infectious particles and cannot cause influenza and (2) FLUCELVAX QUADRIVALENT is intended to provide protection against illness due to influenza viruses only and cannot provide protection against other respiratory illnesses.
Instruct vaccine recipients to report adverse reactions to their healthcare provider.
Provide vaccine recipients with the Vaccine Information Statements which are required by the National Childhood Vaccine Injury Act of 1986. These materials are available free of charge at the Centers for Disease Control and Prevention (CDC) website (www.cdc.gov/vaccines).
Inform vaccine recipients that annual vaccination is recommended.
FLUCELVAX QUADRIVALENT is a registered trademark of Seqirus UK Limited or its affiliates.
Manufactured by: Seqirus Inc. Holly Springs, NC 27540, USA
US License No. 2049
Distributed by: Seqirus USA Inc. 25 Deforest Avenue, Summit, NJ 07901, USA
1-855-358-8966
Principal Display Panel – 5 mL Carton Label
NDC 70461-421-10
5mL vial
Rx Only
Seqirus™
For use in persons 6 months of age and older
For Intramuscular Injection Only
Influenza Vaccine
FLUCELVAX®
QUADRIVALENT
2021 - 2022 Formula
Principal Display Panel – 5 mL Vial Label
NDC 70461-421-11
Influenza Vaccine
FLUCELVAX®
QUADRIVALENT
2021 - 2022 Formula
5 mL Multi-dose Vial
| FLUCELVAX QUADRIVALENT
(PRE-FILLED SYRINGE)
influenza a virus a/washington/19/2020 (h1n1) antigen (mdck cell derived, propiolactone inactivated), influenza a virus a/tasmania/503/2020 (h3n2) antigen (mdck cell derived, propiolactone inactivated), influenza b virus b/darwin/7/2019 antigen (mdck cell derived, propiolactone inactivated), influenza b virus b/singapore/inftt-16-0610/2016 antigen (mdck cell derived, propiolactone inactivated) injection, suspension |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| FLUCELVAX QUADRIVALENT
(MULTI-DOSE VIAL)
influenza a virus a/washington/19/2020 (h1n1) antigen (mdck cell derived, propiolactone inactivated), influenza a virus a/tasmania/503/2020 (h3n2) antigen (mdck cell derived, propiolactone inactivated), influenza b virus b/darwin/7/2019 antigen (mdck cell derived, propiolactone inactivated), influenza b virus b/singapore/inftt-16-0610/2016 antigen (mdck cell derived, propiolactone inactivated) injection, suspension |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Seqirus Inc. (080102141) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Seqirus Inc. | 080102141 | API MANUFACTURE, MANUFACTURE, LABEL, PACK, ANALYSIS | |