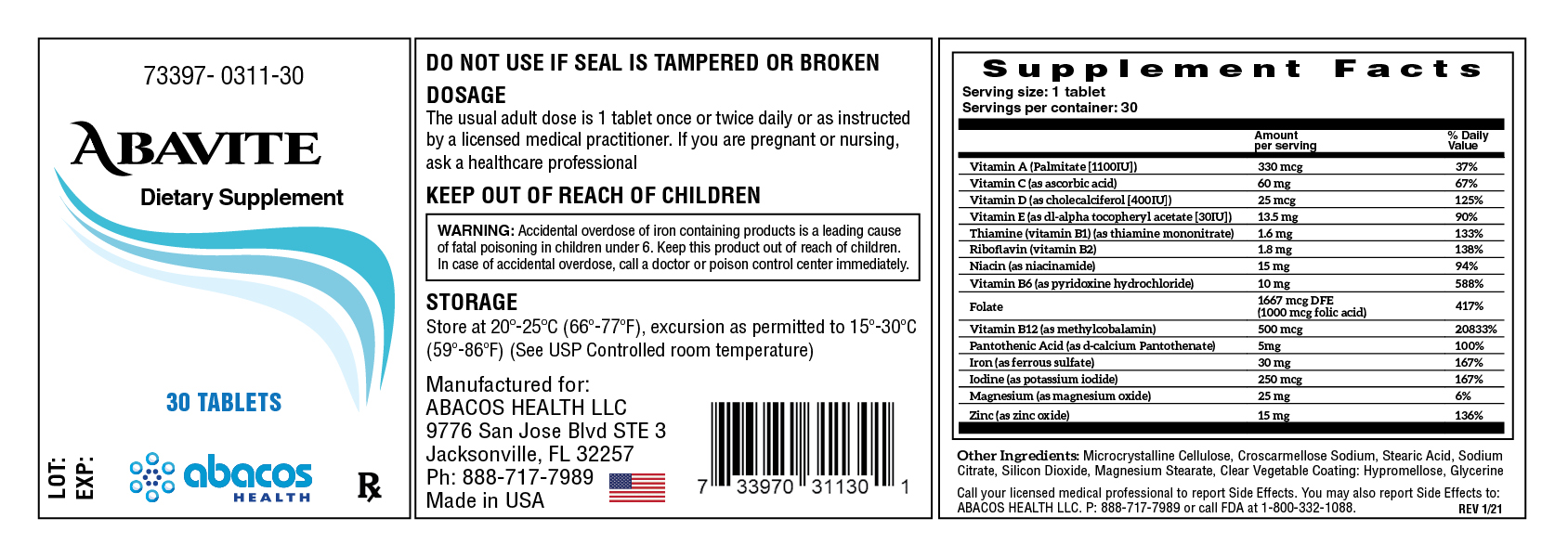
ABAVITE- vitamin a, vitamin c, vitamin d, vitamin e, thiamine, riboflavin, niacin, vitamin b6, folate, vitamin b12, pantothenic acid, iron, iodine, magnesium, zinc. tablet
ABACOS HEALTH
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
]
|
Name of Ingredient |
Amount per serving |
% Daily Value |
|
Vitamin A (Palmitate [1100IU] |
330 mcg |
37% |
|
Vitamin C (as ascorbic acid) |
60 mg |
67% |
|
Vitamin D (as cholecalciferol [400IU]) |
25 mcg |
125% |
|
Vitamin E (as dl-alpha tocopheryl acetate [30IU]) |
13.5 mg |
90% |
|
Thiamine (vitamin B1) (as thiamine mononitrate) |
1.6 mg |
133% |
|
Riboflavin (vitamin B2) |
1.8 mg |
138% |
|
Niacin (as niacinamide) |
15 mg |
94% |
|
Vitamin B6 (as pyridoxine hydrochloride) |
10 mg |
588% |
|
Folate |
1667 mcg DFE (1000 mcg folic acid) |
417% |
|
Vitamin B12 (as methylcobalamin) |
500 mcg |
20833% |
|
Pantothenic acid (as d-calcium pantothenate) |
5 mg |
100% |
|
Iron (as ferrous sulfate) |
30 mg |
167% |
|
Iodine (as potassium iodide) |
250 mcg |
167% |
|
Magnesium (as magnesium oxide) |
25 mg |
6% |
|
Zinc (as zinc oxide) |
15 mg |
136% |
Other inactive Ingredients:
Other Ingredients: Microcrystalline Cellulose, Croscarmellose Sodium, Stearic Acid, SodiumCitrate, Silicon Dioxide, Magnesium Stearate, Clear Vegetable Coating: Hypromellose, Glycerine
ABAVITE is a supplement indicated to provide vitamin, mineral supplementation to support optimum vitamin levels.
The usual adult dose is 1 tablet once or twice daily or as instructed by a licensed medical practitioner. If you are pregnant or nursing,ask a healthcare professional
Vitamin D therapy should be cautiously used in patients with hyperparathyroidism (or any condition which may lead directly or indirectly to hypercalcemia), hypercalcemia, or people who have renal stones or at risk of having renal stones. High doses of vitamin D may elevate levels of calcium, which may eventually accumulate in the blood and soft tissues. Bone pain, high blood pressure, the formation of kidney stones, renal failure, and increased risk of heart disease can also occur.
Prolonged use of iron supplements may lead to iron storage disease.
WARNING: Accidental overdose of iron containing products is a leading cause of fatal poisoning in children under 6. Keep this product out of reach of children. In case of accidental overdose, call a doctor or poison control center immediately.
High Dose Folic acid supplementation may conceal an occult vitamin B 12 deficiency and further exacerbate or initiate neurologic disease. Therefore, clinicians should consider ruling out vitamin B 12 deficiency before initiating folic acid therapy.
Do NOT use Abavite if you have an allergy for one or more components listed above.
Possible interactions:
High doses of folic acid may result in reduced serum levels of anticonvulsant drugs.
Vitamin D supplementation should be avoided in hypercalcemia or conditions that may lead to hypercalcemia such as hyperparathyroidism and those who form calcium-containing kidney stones (Calcium oxalate stones).
Consult your physician or pharmacist for additional information regarding vitamin-drug interactions.
ABAVITE is Contraindicated in patients with known or documented hypersensitivity to any of its listed ingredients or color additives. Folic acid is a contraindication in patients with untreated and uncomplicated pernicious anemia and those with an anaphylactic allergy to folic acid. Iron therapy is a contraindication in patients with hemochromatosis or Potential or current disease, which involves iron storage due to chronic hemolytic anemia (e.g., inherited anomalies of hemoglobin structure or synthesis), red cell enzyme deficiencies, etc.); pyridoxine responsive anemia, or cirrhosis of the liver. Methylcobalamin is a contraindication in patients with sensitivity to cobalt or methylcobalamin (Vitamin B-12).
| ABAVITE
vitamin a, vitamin c, vitamin d, vitamin e, thiamine, riboflavin, niacin, vitamin b6, folate, vitamin b12, pantothenic acid, iron, iodine, magnesium, zinc. tablet |
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - ABACOS HEALTH (117194224) |