Label: metoprolol tartrate- Metoprolol tartrate injection
-
Contains inactivated NDC Code(s)
NDC Code(s): 0591-3195-26, 0591-3195-78 - Packager: Watson laboratories, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated April 7, 2006
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- N/A - Section Title Not Found In Database
-
DESCRIPTION
Metoprolol tartrate injection is a sterile solution containing metoprolol tartrate, a selective beta1-adrenoreceptor blocking agent, available for intravenous administration. Metoprolol tartrate is 1-(isopropylamino)-3-[p-(2-methoxyethyl)phenoxy]-2-propanol (2:1) dextro-tartrate salt. Its structural formula is:
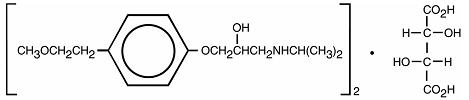
(C15H25NO3)2 -C4H6O6
Metoprolol tartrate is a white, crystalline powder with a molecular weight of 684.82. It is very soluble in water; freely soluble in methylene chloride, in chloroform, and in alcohol; slightly soluble in acetone; and insoluble in ether.
Each 5 mL vial contains: Metoprolol Tartrate 5 mg, Sodium Chloride 45 mg, in Water for Injection q. s.
-
CLINICAL PHARMACOLOGY
Metoprolol tartrate is a beta-adrenergic receptor blocking agent. In vitro and in vivo animal studies have shown that it has a preferential effect on beta1 adrenoreceptors, chiefly located in cardiac muscle. This preferential effect is not absolute, however, and at higher doses, metoprolol also inhibits beta2 adrenoreceptors, chiefly located in the bronchial and vascular musculature.
Clinical pharmacology studies have confirmed the beta-blocking activity of metoprolol in man, as shown by (1) reduction in heart rate and cardiac output at rest and upon exercise, (2) reduction of systolic blood pressure upon exercise, (3) inhibition of isoproterenol-induced tachycardia, and (4) reduction of reflex orthostatic tachycardia.
Relative beta1 selectivity has been confirmed by the following: (1) In normal subjects, metoprolol is unable to reverse the beta2-mediated vasodilating effects of epinephrine. This contrasts with the effect of nonselective (beta1 plus beta2) beta blockers, which completely reverse the vasodilating effects of epinephrine. (2) In asthmatic patients, metoprolol reduces FEV1 and FVC significantly less than a nonselective beta blocker, propranolol, at equivalent beta1-receptor blocking doses.
Metoprolol has no intrinsic sympathomimetic activity, and membrane-stabilizing activity is detectable only at doses much greater than required for beta blockade. Metoprolol crosses the blood-brain barrier and has been reported in the CSF in a concentration 78% of the simultaneous plasma concentration. Animal and human experiments indicate that metoprolol slows the sinus rate and decreases AV nodal conduction.
In a large (1,395 patients randomized), double-blind, placebo-controlled clinical study, metoprolol was shown to reduce 3-month mortality by 36% in patients with suspected or definite myocardial infarction.
Patients were randomized and treated as soon as possible after their arrival in the hospital, once their clinical condition had stabilized and their hemodynamic status had been carefully evaluated.
Subjects were ineligible if they had hypotension, bradycardia, peripheral signs of shock, and/or more than minimal basal rales as signs of congestive heart failure. Initial treatment consisted of intravenous followed by oral administration of metoprolol tartrate or placebo, given in a coronary care or comparable unit. Oral maintenance therapy with metoprolol or placebo was then continued for 3 months. After this double-blind period, all patients were given metoprolol and followed up to 1 year.
The median delay from the onset of symptoms to the initiation of therapy was 8 hours in both the metoprolol and placebo treatment groups. Among patients treated with metoprolol, there were comparable reductions in 3-month mortality for those treated early (≤ 8 hours) and those in whom treatment was started later. Significant reductions in the incidence of ventricular fibrillation and in chest pain following initial intravenous therapy were also observed with metoprolol and were independent of the interval between onset of symptoms and initiation of therapy.
The precise mechanism of action of metoprolol in patients with suspected or definite myocardial infarction is not known.
In this study, patients treated with metoprolol received the drug both very early (intravenously) and during a subsequent 3-month period, while placebo patients received no beta blocker treatment for this period. The study thus was able to show a benefit from the overall metoprolol regimen but cannot separate the benefit of very early intravenous treatment from the benefit of later beta blocker therapy. Nonetheless, because the overall regimen showed a clear beneficial effect on survival without evidence of an early adverse effect on survival, one acceptable dosage regimen is the precise regimen used in the trial. Because the specific benefit of very early treatment remains to be defined however, it is also reasonable to administer the drug orally to patients at a later time as is recommended for certain other beta blockers.
Pharmacokinetics
In man, absorption of metoprolol is rapid and complete. Plasma levels following oral administration, however, approximate 50% of levels following intravenous administration, indicating about 50% first-pass metabolism.
Plasma levels achieved are highly variable after oral administration. Only a small fraction of the drug (about 12%) is bound to human serum albumin. Elimination is mainly by biotransformation in the liver, and the plasma half-life ranges from approximately 3 to 7 hours. The systemic availability and half-life of metoprolol in patients with renal failure do not differ to a clinically significant degree from those in normal subjects. Consequently, no reduction in dosage is usually needed in patients with chronic renal failure.
Following intravenous administration of metoprolol, the urinary recovery of unchanged drug is approximately 10%. When the drug was infused over a 10 minute period, in normal volunteers, maximum beta blockade was achieved at approximately 20 minutes. Doses of 5 mg and 15 mg yielded a maximal reduction in exercise-induced heart rate of approximately 10% and 15%, respectively. The effect on exercise heart rate decreased linearly with time at the same rate for both doses, and disappeared at approximately 5 hours and 8 hours for the 5 mg and 15 mg doses, respectively.
Equivalent maximal beta-blocking effect is achieved with oral and intravenous doses in the ratio of approximately 2.5:1.
There is a linear relationship between the log of plasma levels and reduction of exercise heart rate.
In several studies of patients with acute myocardial infarction, intravenous followed by oral administration of metoprolol caused a reduction in heart rate, systolic blood pressure, and cardiac output. Stroke volume, diastolic blood pressure, and pulmonary artery end diastolic pressure remained unchanged.
-
INDICATIONS AND USAGE
Myocardial Infarction
Metoprolol tartrate injection and tablets are indicated in the treatment of hemodynamically stable patients with definite or suspected acute myocardial infarction to reduce cardiovascular mortality. Treatment with intravenous metoprolol tartrate can be initiated as soon as the patient’s clinical condition allows (see DOSAGE AND ADMINISTRATION, CONTRAINDICATIONS, and WARNINGS). Alternatively, treatment can begin within 3 to 10 days of the acute event (see DOSAGE AND ADMINISTRATION).
-
CONTRAINDICATIONS
Myocardial Infarction
Metoprolol is contraindicated in patients with a heart rate < 45 beats/min; second- and third-degree heart block; significant first-degree heart block (P-R interval ≥ 0.24 sec); systolic blood pressure < 100 mmHg; or moderate-to-severe cardiac failure (see WARNINGS).
-
WARNINGS
Myocardial Infarction
Cardiac Failure:
Sympathetic stimulation is a vital component supporting circulatory function, and beta blockade carries the potential hazard of depressing myocardial contractility and precipitating or exacerbating minimal cardiac failure.
During treatment with metoprolol, the hemodynamic status of the patient should be carefully monitored. If heart failure occurs or persists despite appropriate treatment, metoprolol should be discontinued.
Bradycardia:
Metoprolol produces a decrease in sinus heart rate in most patients; this decrease is greatest among patients with high initial heart rates and least among patients with low initial heart rates. Acute myocardial infarction (particularly inferior infarction) may in itself produce significant lowering of the sinus rate. If the sinus rate decreases to < 40 beats/min, particularly if associated with evidence of lowered cardiac output, atropine (0.25-0.5 mg) should be administered intravenously. If treatment with atropine is not successful, metoprolol should be discontinued, and cautious administration of isoproterenol or installation of a cardiac pacemaker should be considered.
AV Block:
Metoprolol slows AV conduction and may produce significant first-(P-R interval ≥0.26 sec), second-, or third-degree heart block. Acute myocardial infarction also produces heart block.
If heart block occurs, metoprolol should be discontinued and atropine (0.25-0.5 mg) should be administered intravenously. If treatment with atropine is not successful, cautious administration of isoproterenol or installation of a cardiac pacemaker should be considered.
Hypotension:
If hypotension (systolic blood pressure ≤90 mmHg) occurs, metoprolol should be discontinued, and the hemodynamic status of the patient and the extent of myocardial damage carefully assessed. Invasive monitoring of central venous, pulmonary capillary wedge, and arterial pressures may be required. Appropriate therapy with fluids, positive inotropic agents, balloon counterpulsation, or other treatment modalities should be instituted. If hypotension is associated with sinus bradycardia or AV block, treatment should be directed at reversing these (see above).
Bronchospastic Diseases:
PATIENTS WITH BRONCHOSPASTIC DISEASES SHOULD, IN GENERAL, NOT RECEIVE BETA BLOCKERS. Because of its relative beta1 selectivity, metoprolol may be used with extreme caution in patients with bronchospastic disease. Because it is unknown to what extent beta2-stimulating agents may exacerbate myocardial ischemia and the extent of infarction, these agents should not be used prophylactically. If bronchospasm not related to congestive heart failure occurs, metoprolol should be discontinued. A theophylline derivative or a beta2 agonist may be administered cautiously, depending on the clinical condition of the patient. Both theophylline derivatives and beta2 agonists may produce serious cardiac arrhythmias.
-
PRECAUTIONS
Laboratory Tests
Clinical laboratory findings may include elevated levels of serum transaminase, alkaline phosphatase, and lactate dehydrogenase.
Drug Interactions
Catecholamine-depleting drugs (e.g., reserpine) may have an additive effect when given with beta-blocking agents. Patients treated with metoprolol plus a catecholamine depletor should therefore be closely observed for evidence of hypotension or marked bradycardia, which may produce vertigo, syncope, or postural hypotension.
While taking beta blockers, patients with a history of severe anaphylactic reaction to a variety of allergens may be more reactive to repeated challenge, either accidental, diagnostic or therapeutic. Such patients may be unresponsive to the usual doses of epinephrine used to treat allergic reaction.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals have been conducted to evaluate carcinogenic potential. In a 2-year study in rats at three oral dosage levels of up to 800 mg/kg per day, there was no increase in the development of spontaneously occurring benign or malignant neoplasms of any type. The only histologic changes that appeared to be drug related were an increased incidence of generally mild focal accumulation of foamy macrophages in pulmonary alveoli and a slight increase in biliary hyperplasia. In a 21-month study in Swiss albino mice at three oral dosage levels of up to 750 mg/kg per day, benign lung tumors (small adenomas) occurred more frequently in female mice receiving the highest dose than in untreated control animals. There was no increase in malignant or total (benign plus malignant) lung tumors, nor in the overall incidence of tumors or malignant tumors. This 21-month study was repeated in CD-1 mice, and no statistically or biologically significant differences were observed between treated and control mice of either sex for any type of tumor.
All mutagenicity tests performed (a dominant lethal study in mice, chromosome studies in somatic cells, a Salmonella/mammalian-microsome mutagenicity test, and a nucleus anomaly test in somatic interphase nuclei) were negative.
No evidence of impaired fertility due to metoprolol was observed in a study performed in rats at doses up to 55.5 times the maximum daily human dose of 450 mg.
Pregnancy Category C
Metoprolol has been shown to increase postimplantation loss and decrease neonatal survival in rats at doses up to 55.5 times the maximum daily human dose of 450 mg. Distribution studies in mice confirm exposure of the fetus when metoprolol is administered to the pregnant animal. These studies have revealed no evidence of impaired fertility or teratogenicity. There are no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
-
ADVERSE REACTIONS
Myocardial Infarction
Central Nervous System:
Tiredness has been reported in about 1 of 100 patients. Vertigo, sleep disturbances, hallucinations, headache, dizziness, visual disturbances, confusion, and reduced libido have also been reported, but a drug relationship is not clear.
Cardiovascular:
In the randomized comparison of metoprolol and placebo described in the CLINICAL PHARMACOLOGY section, the following adverse reactions were reported:
Metoprolol Placebo Hypotension 27.4% 23.2% (systolic BP < 90 mmHg) Bradycardia 15.9% 6.7% (heart rate < 40 beats/min) Second- or third-degree heart block 4.7% 4.7% First-degree heart block 5.3% 1.9% (P-R ≥ 0.26 sec) Heart failure 27.5% 29.6% Respiratory:
Dyspnea of pulmonary origin has been reported in fewer than 1 of 100 patients.
Gastrointestinal:
Nausea and abdominal pain have been reported in fewer than 1 of 100 patients.
Dermatologic:
Rash and worsened psoriasis have been reported, but a drug relationship is not clear.
Miscellaneous:
Unstable diabetes and claudication have been reported, but a drug relationship is not clear.
Potential Adverse Reactions
A variety of adverse reactions not listed above have been reported with other beta-adrenergic blocking agents and should be considered potential adverse reactions to metoprolol.
Central Nervous System:
Reversible mental depression progressing to catatonia; an acute reversible syndrome characterized by disorientation for time and place, short-term memory loss, emotional lability, slightly clouded sensorium, and decreased performance on neuropsychometrics.
Cardiovascular:
Intensification of AV block (see CONTRAINDICATIONS).
Hematologic:
Agranulocytosis, nonthrombocytopenic purpura, thrombocytopenic purpura.
Hypersensitive Reactions:
Fever combined with aching and sore throat, laryngospasm, and respiratory distress.
-
OVERDOSAGE
Acute Toxicity
Several cases of overdosage have been reported, some leading to death.
Oral LD50’s (mg/kg): mice, 1158-2460; rats, 3090-4670.
Signs and Symptoms
Potential signs and symptoms associated with overdosage with metoprolol are bradycardia, hypotension, bronchospasm, and cardiac failure.
Treatment
There is no specific antidote.
In general, patients with acute or recent myocardial infarction may be more hemo-dynamically unstable than other patients and should be treated accordingly (see WARNINGS, Myocardial Infarction).
On the basis of the pharmacologic actions of metoprolol, the following general measures should be employed:
Elimination of the Drug:
Gastric lavage should be performed.
Bradycardia:
Atropine should be administered. If there is no response to vagal blockade, isoproterenol should be administered cautiously.
Hypotension:
A vasopressor should be administered, e.g., norepinephrine or dopamine.
Bronchospasm:
A beta2-stimulating agent and/or a theophylline derivative should be administered.
Cardiac Failure: A digitalis glycoside and diuretic should be administered. In shock resulting from inadequate cardiac contractility, administration of dobutamine, isoproterenol, or glucagon may be considered.
-
DOSAGE AND ADMINISTRATION
Myocardial Infarction
Early Treatment:
During the early phase of definite or suspected acute myocardial infarction, treatment with metoprolol tartrate can be initiated as soon as possible after the patient’s arrival in the hospital. Such treatment should be initiated in a coronary care or similar unit immediately after the patient’s hemodynamic condition has stabilized.
Treatment in this early phase should begin with the intravenous administration of three bolus injections of 5 mg of metoprolol tartrate each; the injections should be given at approximately 2-minute intervals. During the intravenous administration of metoprolol, blood pressure, heart rate, and electrocardiogram should be carefully monitored.
In patients who tolerate the full intravenous dose (15 mg), metoprolol tartrate tablets, 50 mg every 6 hours, should be initiated 15 minutes after the last intravenous dose and continued for 48 hours. Thereafter, patients should receive a maintenance dosage of 100 mg twice daily (see Late Treatment below).
Patients who appear not to tolerate the full intravenous dose should be started on metoprolol tartrate tablets either 25 mg or 50 mg every 6 hours (depending on the degree of intolerance) 15 minutes after the last intravenous dose or as soon as their clinical condition allows. In patients with severe intolerance, treatment with metoprolol should be discontinued (see WARNINGS).
Late Treatment:
Patients with contraindications to treatment during the early phase of suspected or definite myocardial infarction, patients who appear not to tolerate the full early treatment, and patients in whom the physician wishes to delay therapy for any other reason should be started on metoprolol tartrate tablets, 100 mg twice daily, as soon as their clinical condition allows. Therapy should be continued for at least 3 months. Although the efficacy of metoprolol beyond 3 months has not been conclusively established, data from studies with other beta blockers suggest that treatment should be continued for 1 to 3 years.
NOTE: Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
-
HOW SUPPLIED
Metoprolol Tartrate Injection USP, 1 mg/mL is available in 5 mL single dose vials, in cartons of 3.
Store at controlled room temperature 15°-30°C (59°-86°F).
PROTECT FROM LIGHT. Store in carton until time of use.
Literature revised: December 2002
Product No.: 0958-05
695309580591*A1
Watson Laboratories, Inc.
Corona, CA 92880 USA
-
INGREDIENTS AND APPEARANCE
METOPROLOL TARTRATE
metoprolol tartrate injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0591-3195 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Metoprolol tartrate (UNII: W5S57Y3A5L) (Metoprolol - UNII:GEB06NHM23) 1 mg in 1 mL Inactive Ingredients Ingredient Name Strength Sodium Chloride (UNII: 451W47IQ8X) 9 mg in 1 mL Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0591-3195-78 3 in 1 CARTON 1 NDC:0591-3195-26 5 mL in 1 VIAL, SINGLE-DOSE Labeler - Watson laboratories, Inc.
