TRAUMED- arnica root tablet
MediNatura Inc.
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Traumed Tablet
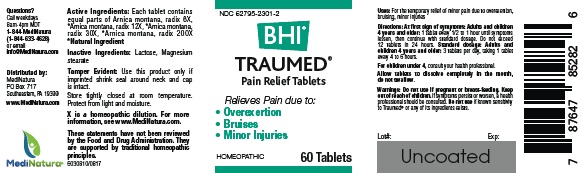
WARNINGS
If pregnant or breast-feeding, ask a health professional before use. If symptoms persist or worsen, a healthcare professional should be consulted. Do not use if known sensitivity to Traumed® or any of its ingredients exists.
DOSAGE
Directions: At first sign of symptoms:Adults and children 4 years and older:
1 tablet every 1/2 to 1 hour until symptoms lessen, then continue with standard dosage. Do not exceed 12 tablets in 24 hours.
Standard dosage:
Adults and children 4 years and older:
3 tablets per day, taking 1 tablet every 4 to 6 hours.
Allow tablets to dissolve completely in the mouth, do not swallow
| TRAUMED
arnica root tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - MediNatura Inc. (079324099) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| MediNatura Inc. | 102783016 | manufacture(62795-2301) | |