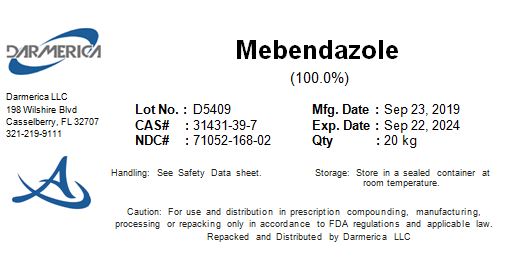
MEBENDAZOLE- mebendazole powder
DARMERICA, LLC
----------
Mebendazole
| MEBENDAZOLE
mebendazole powder |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - DARMERICA, LLC (080233052) |
Revised: 12/2021
Document Id: d2a658e0-08dc-a9a1-e053-2a95a90aa014
Set id: ab0c6056-9ab3-febe-e053-2a95a90aecc7
Version: 3
Effective Time: 20211208
DARMERICA, LLC