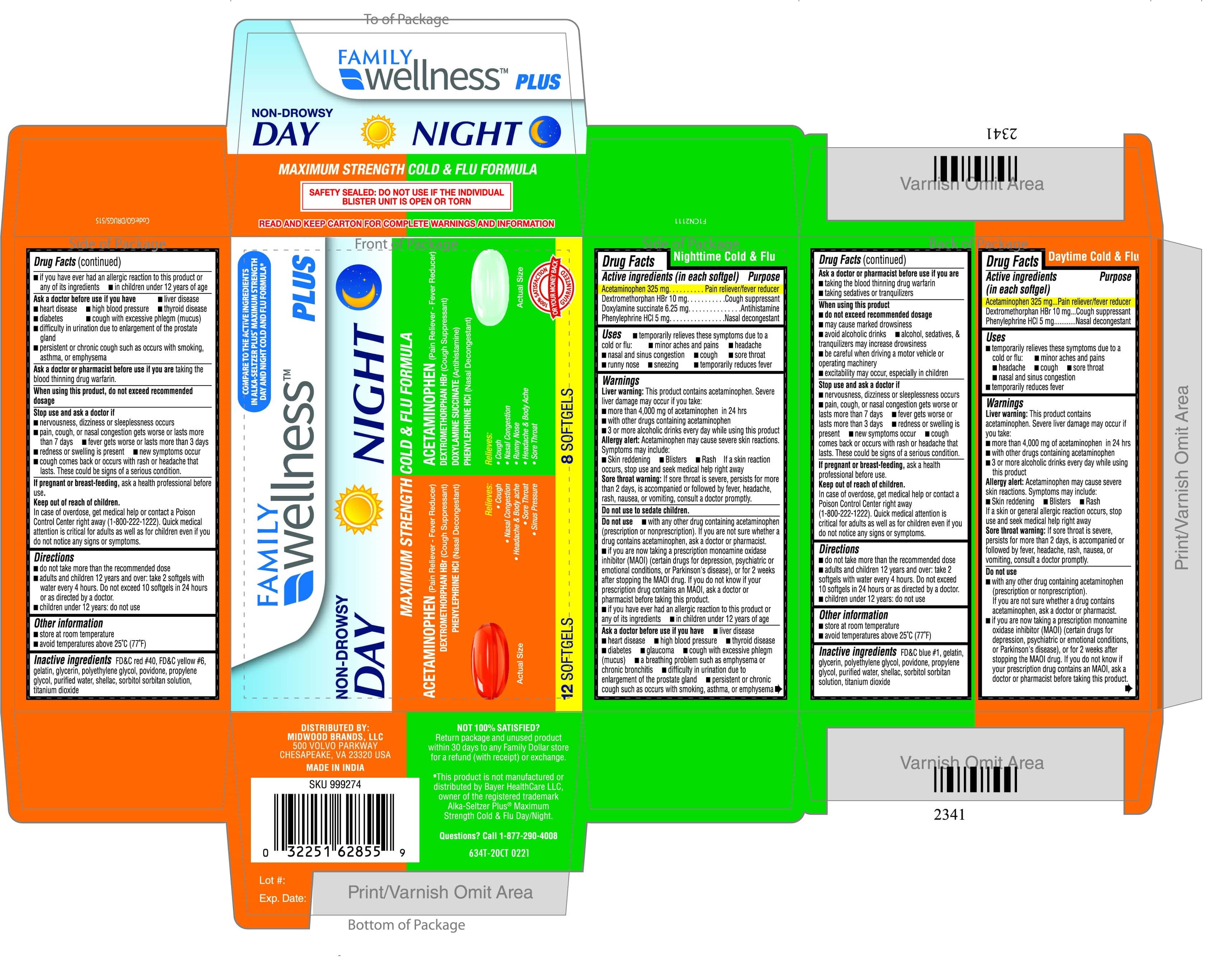
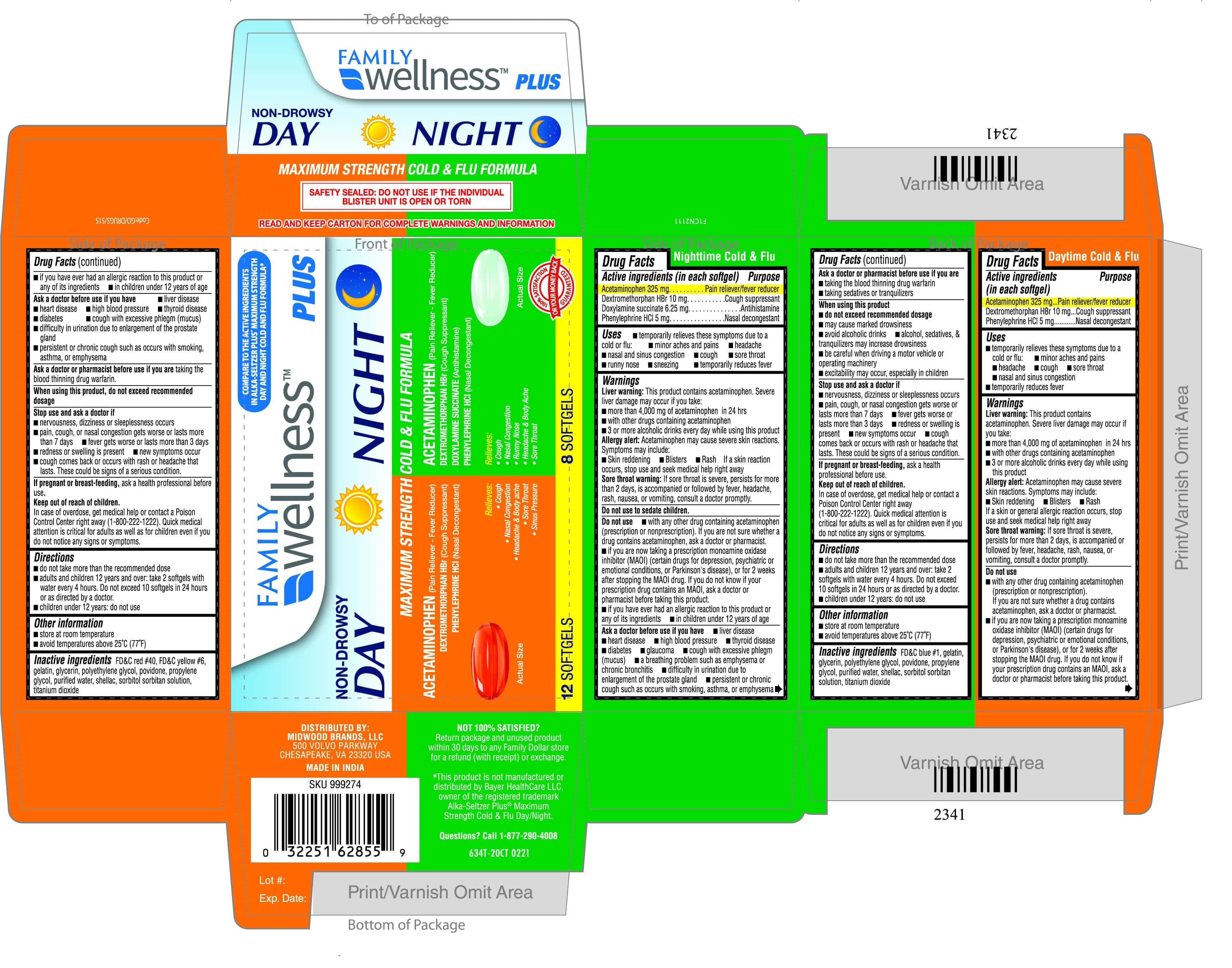
Label: MAXIMUM STRENGTH DAY-NIGHT COLD AND FLU FORMULA- acetaminophen, dextromethorphan, phenylephrine hcl kit
- NDC Code(s): 55319-933-22, 55319-934-08, 55319-935-02
- Packager: FAMILY DOLLAR
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 26, 2021
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DRUG FACTS - DAY MAXIMUM STRENGTH COLD AND FLU (633T)
- ACTIVE INGREDIENT
- INACTIVE INGREDIENT
- PURPOSE
- DOSAGE & ADMINISTRATION
- INDICATIONS & USAGE
-
WARNINGS
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
· more than 4,000 mg of acetaminophen in 24 hours
· with other drugs containing acetaminophen
· 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin or severe
allergic reactions. Symptoms may include:
· skin reddening · blisters · rash · hives
· facial swelling · asthma (wheezing) · shock
If a skin or general allergic reaction occurs, stop use and seek medical help right away.
Sore throat warning: If sore throat is severe, persists for more than
2 days, is accompanied or followed by fever, headache, rash, nausea,
or vomiting, consult a doctor promptly.
- KEEP OUT OF REACH OF CHILDREN
- DRUG FACTS - NIGHT MAXIMUM STRENGTH COLD AND FLU
- ACTIVE INGREDIENT
- INACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- DOSAGE & ADMINISTRATION
- KEEP OUT OF REACH OF CHILDREN
-
WARNINGS
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
· more than 4,000 mg of acetaminophen in 24 hours
· with other drugs containing acetaminophen
· 3 or more aloholic drinks every day while using this product
l Allergy alert: Acetaminophen may cause severe skin or severe
allergic reactions. Symptoms may include:
· skin reddening · blisters · rash · hives
· facial swelling · asthma (wheezing) · shock
If a skin or general allergic reaction occurs, stop use and seek medical help right away.
Sore throat warning: If sore throat is severe, persists for more than
2 days, is accompanied or followed by fever, headache, rash, nausea,
or vomiting, consult a doctor promptly.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MAXIMUM STRENGTH DAY-NIGHT COLD AND FLU FORMULA
acetaminophen, dextromethorphan, phenylephrine hcl kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:55319-935 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55319-935-02 1 in 1 BLISTER PACK; Type 0: Not a Combination Product 08/02/2020 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BLISTER PACK 12 Part 2 1 BLISTER PACK 8 Part 1 of 2 MAXIMUM STRENGHT DAY COLD AND FLU FORMULA
acetaminophen, dextromethorphan, phenylephrine hcl capsule, liquid filledProduct Information Item Code (Source) NDC:55319-933 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 325 mg PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 5 mg DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 10 mg Inactive Ingredients Ingredient Name Strength SHELLAC (UNII: 46N107B71O) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) FD&C RED NO. 40 (UNII: WZB9127XOA) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SORBITOL (UNII: 506T60A25R) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POVIDONE (UNII: FZ989GH94E) GELATIN (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) Product Characteristics Color orange Score no score Shape OVAL Size 16mm Flavor Imprint Code 70 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55319-933-22 12 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 08/20/2020 Part 2 of 2 MAXIMUM STRENTH NIGHT COLD AND FLU FORMULA
acetaminophen, dextromethorphan hbr, doxylamine succinate, phenylephrine hcl capsule, liquid filledProduct Information Item Code (Source) NDC:55319-934 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 10 mg PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 5 mg ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 325 mg DOXYLAMINE SUCCINATE (UNII: V9BI9B5YI2) (DOXYLAMINE - UNII:95QB77JKPL) DOXYLAMINE SUCCINATE 6.25 mg Inactive Ingredients Ingredient Name Strength SORBITOL (UNII: 506T60A25R) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POVIDONE (UNII: FZ989GH94E) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) GELATIN (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) SHELLAC (UNII: 46N107B71O) Product Characteristics Color green Score no score Shape OVAL Size 16mm Flavor Imprint Code 72 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55319-934-08 8 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 08/20/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 08/02/2020 Labeler - FAMILY DOLLAR (024472631) Registrant - TIME CAP LABORATORIES, INC. (037052099) Establishment Name Address ID/FEI Business Operations MARKSANS PHARMA LTD 925822975 manufacture(55319-935)