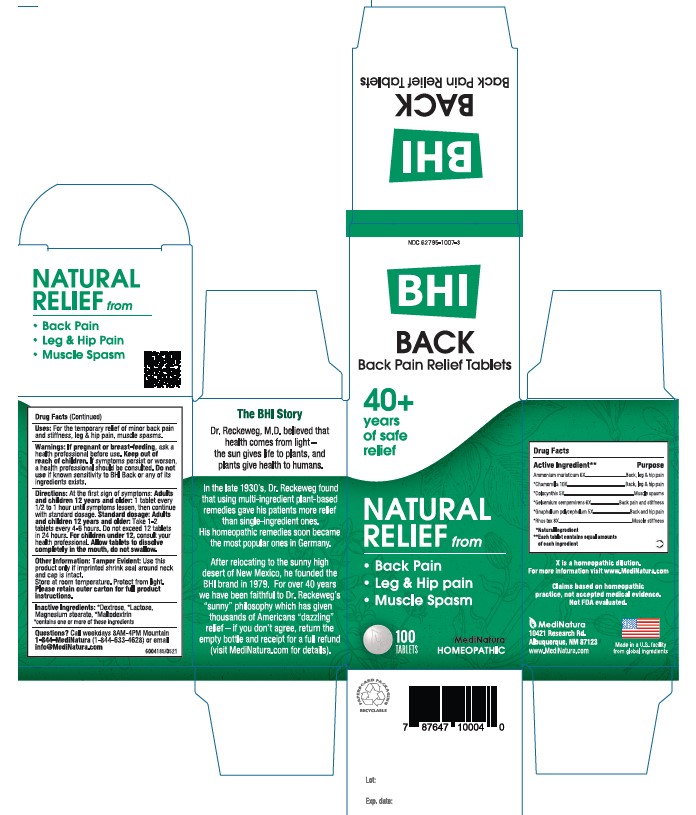
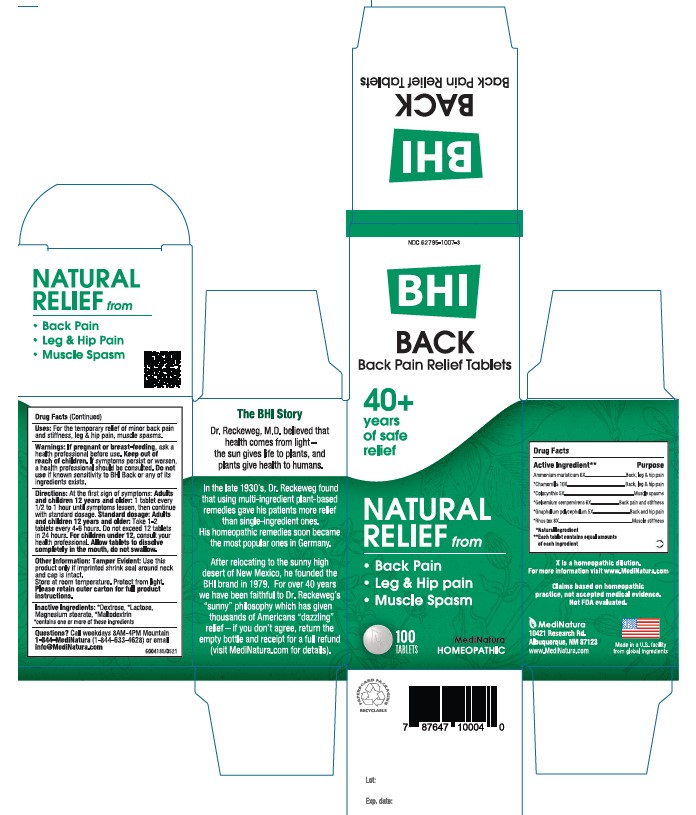
Label: BHI BACK- ammonium chloride, matricaria recutita,citrullus colocynthis fruit pulp, pseudognaphalium obtusifolium, and toxicodendron pubescens leaf tablet
- NDC Code(s): 62795-1007-2, 62795-1007-3
- Packager: MediNatura Inc
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated May 23, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- INACTIVE INGREDIENT
- PURPOSE
- USES
-
DIRECTIONS
At first sign of symptoms:
Adults and children 12 years and older: 1 tablet every 1/2 to 1 hour until symptoms lessen, then continue with standard dosage.
Standard dosage: Adults and children 12 years and older: take 1-2 tablets 4 to 6 hours. Do not exceed 12 tablets in 24 hours. For children under 12, consult your health professional.
Allow tablets to dissolve completely in the mouth, do not swallow.
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BHI BACK
ammonium chloride, matricaria recutita,citrullus colocynthis fruit pulp, pseudognaphalium obtusifolium, and toxicodendron pubescens leaf tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62795-1007 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AMMONIUM CHLORIDE (UNII: 01Q9PC255D) (AMMONIUM CATION - UNII:54S68520I4) AMMONIUM CATION 6 [hp_X] MATRICARIA RECUTITA (UNII: G0R4UBI2ZZ) (MATRICARIA RECUTITA - UNII:G0R4UBI2ZZ) MATRICARIA RECUTITA 10 [hp_X] CITRULLUS COLOCYNTHIS FRUIT PULP (UNII: 23H32AOH17) (CITRULLUS COLOCYNTHIS FRUIT PULP - UNII:23H32AOH17) CITRULLUS COLOCYNTHIS FRUIT PULP 5 [hp_X] GELSEMIUM SEMPERVIRENS ROOT (UNII: 639KR60Q1Q) (GELSEMIUM SEMPERVIRENS ROOT - UNII:639KR60Q1Q) GELSEMIUM SEMPERVIRENS ROOT 6 [hp_X] PSEUDOGNAPHALIUM OBTUSIFOLIUM (UNII: 36XQ854NWW) (PSEUDOGNAPHALIUM OBTUSIFOLIUM - UNII:36XQ854NWW) PSEUDOGNAPHALIUM OBTUSIFOLIUM 5 [hp_X] TOXICODENDRON PUBESCENS LEAF (UNII: 6IO182RP7A) (TOXICODENDRON PUBESCENS LEAF - UNII:6IO182RP7A) TOXICODENDRON PUBESCENS LEAF 8 [hp_X] Inactive Ingredients Ingredient Name Strength LACTOSE (UNII: J2B2A4N98G) MAGNESIUM STEARATE (UNII: 70097M6I30) DEXTROSE (UNII: IY9XDZ35W2) MALTODEXTRIN (UNII: 7CVR7L4A2D) Product Characteristics Color white Score no score Shape ROUND Size 9mm Flavor Imprint Code Leafman Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62795-1007-3 1 in 1 CARTON 08/30/2014 1 100 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:62795-1007-2 100 in 1 BOTTLE; Type 0: Not a Combination Product 05/23/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/30/2014 Labeler - MediNatura Inc (079324099) Establishment Name Address ID/FEI Business Operations MediNatura Inc 102783016 manufacture(62795-1007)