Active ingredient
Terbinafine hydrochloride 1%
Uses
- •
- cures most athlete's foot (tinea pedis)
- •
- cures most jock itch (tinea cruris) and ringworm (tinea corporis)
- •
- relieves itching, burning, cracking and scaling which accompany these conditions
Warnings
For external use only
Do not use
- •
- on nails or scalp
- •
- in or near the mouth or eyes
- •
- for vaginal yeast infections
When using this product do not get into eyes. If eye contact occurs, rinse thoroughly with water.
Stop use and ask a doctor if too much irritation occurs or gets worse
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- •
- adults and children 12 years and over:
- •
- use the tip of the cap to break the seal and open the tube
- •
- wash the affected skin with soap and water and dry completely before applying
- •
-
for athlete's foot wear well-fitting, ventilated shoes. Change shoes and socks at least once daily.
- •
-
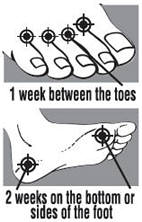
between the toes only: apply twice a day (morning and night) for 1 week or as directed by a doctor
- •
-
on the bottom or sides of the foot: apply twice a day (morning and night) for 2 weeks or as directed by a doctor
- •
-
for jock itch and ringworm: apply once a day (morning or night) for 1 week or as directed by a doctor
- •
- wash hands after each use
- •
- children under 12 years: ask a doctor
|  |
Other information
- •
- do not use if seal on tube is broken or is not visible
- •
- store at controlled room temperature 20°-25°C (68°-77°F)
Inactive ingredients
benzyl alcohol, cetyl alcohol, cetyl palmitate, isopropyl myristate, polysorbate 60, purified water, sodium hydroxide, sorbitan monostearate, stearyl alcohol
Questions or comments?
call 1-800-330-9876
Distributed by: GSK Consumer Healthcare, Warren, NJ 07059
PRINCIPAL DISPLAY PANEL
NDC 0067-8100-30
TERBINAFINE HYDROCHLORIDE CREAM 1% - ANTIFUNGAL
gsk
LAMISILAT ® CREAM
Relieves Itching, Burning, Cracking and Scaling
NET WT 30 g (1 oz)