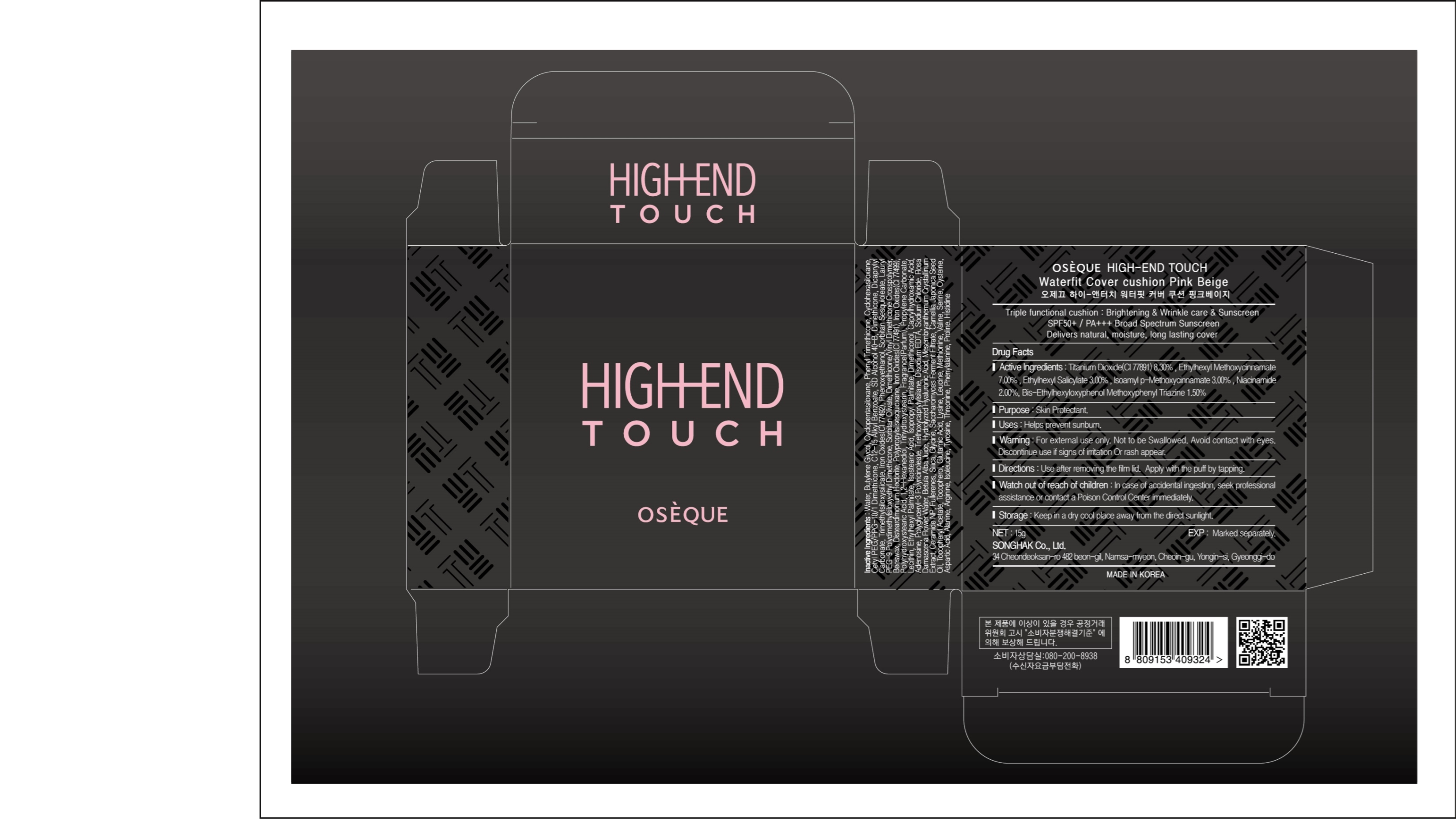
Active ingredients: Titanium Dioxide 8.3%, Ethylhexyl Methoxycinnamate 7%, Ethylhexyl Salicylate 3%, Isoamyl p-Methoxycinnamate 3%, Bis-Ethylhexyloxyphenol Methoxyphenyl Triazine 1.5%, Niacinnamide 2%
Uses: Helps prevent sunburn
Inactive Ingredients: Water, Butylene Glycol, Cyclopentasiloxane, Phenyl Trimethicone, Cyclohexasiloxane, Cetyl PEG/PPG-10/1 Dimethicone, C12-15 Alkyl Benzoate, SD Alcohol 40-B, Dimethicone, Dicaprylyl Carbonate, Iron Oxides (CI 77492), Trimethylsiloxysilicate, Sorbitan Sesquioleate, Phenoxyethanol, Lauryl PEG-9 Polydimethylsiloxyethyl Dimethicone, Sorbitan Olivate, Dimethicone/Vinyl Dimethicone Crosspolymer, Beeswas, Disteardimonium Hectorite, Polypropylsilsesquioxane, Polyhydroxystearic Acid, Iron Oxides (CI 77491), 1,2-Hexanediol, Iron Oxides (CI 77499), Fragrance (Parfum), Propylene Carbonate, Lecithin, Ethylhexyl Palmitate, Isostearic Acid, Isopropyl Palmitate, Dimethiconol, Caprylhydroxamic Acid, Adenosine, Polyglyceryl-3 Polyricinoleate, Triethoxycaprylylsilane, Disodium EDTA, Sodium Chloride, Rosa Damascena Flower Water, Betula Alba Juice, Hydrolyzed Hyaluronic Acid, Silica, Mesembryantheum Crystalinum Extract, Fullerenes, Saccharomyces Ferment Filtrate, Camellia Japonica Seed Oil, Tocopheryl Acetate, Ceramide NP, Glycine, Tocopherol Glutamic Acid, Lysine, Leucine, Methionine, Valine, Serine, Cysteine, Aspartic Acid, Alanine, Arginine, Isoleucine, Tyrosine, Threonine, Phenylalanine, Proline, Histidine
Keep out of reach of children. In case of accidental ingestion, seek professional assistance or contact a Poison Control Center immediately
Warnings: For external use only. Not to be swallowed. Avoid contact with eyes. Discontinue use if signs of irritation or rash appear
Uses: Apply liberally over the face and body 15 minutes prior to sun exposure. Always ensure total coverage of all sun exposed areas. Re-apply every 1-2 hours and always after swimming. For spray type, shake it well before use and spray it evenly at 20cm-30cm apart from face or wherever needed.
Storage: Keep in a dry or room temperature area
SONGHAK CO., LTD.