ALBUMINAR-20- albumin (human) solution
CSL Behring LLC
----------
Albumin (Human) USP, 20%
Albuminar®-20
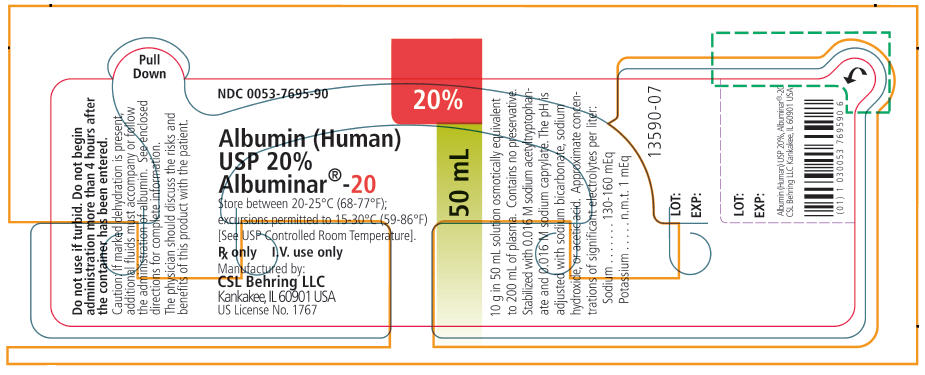
PRINCIPAL DISPLAY PANEL - 50 mL Vial Label
NDC 0053-7695-90
20%
50 mL
Albumin (Human)
USP 20%
Albuminar®-20
Store between 20-25°C (68-77°F);
excursions permitted to 15-30°C (59-86°F)
[See USP Controlled Room Temperature].
Rx only
I.V. use only
Manufactured by:
CSL Behring LLC
Kankakee, IL 60901 USA
US License No. 1767
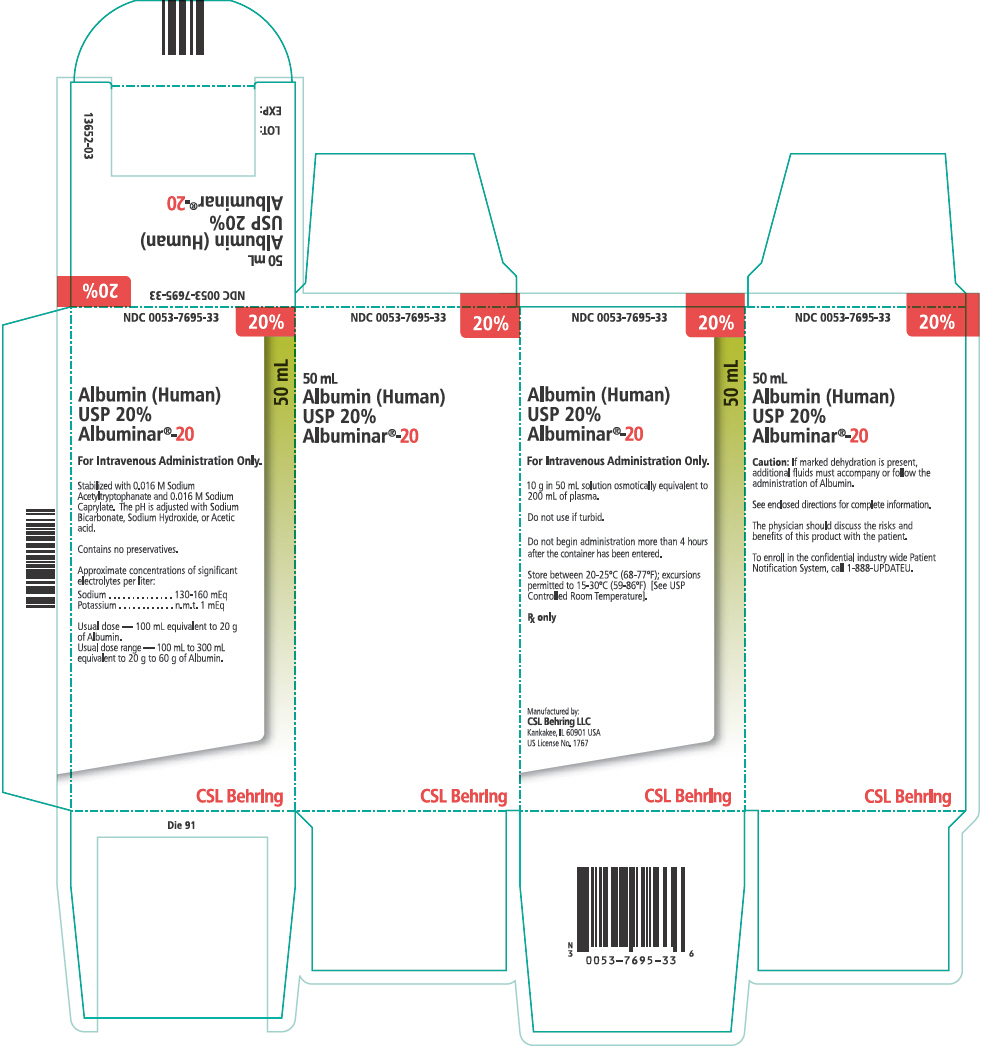
PRINCIPAL DISPLAY PANEL - 50 mL Vial Carton
NDC 0053-7695-33
20%
50 mL
Albumin (Human)
USP 20%
Albuminar®-20
For Intravenous Administration Only.
10 g in 50 mL solution osmotically equivalent to
200 mL of plasma.
Do not use if turbid.
Do not begin administration more than 4 hours
after the container has been entered.
Store between 20-25°C (68-77°F); excursions
permitted to 15-30°C (59-86°F) [See USP
Controlled Room Temperature].
Rx only
Manufactured by:
CSL Behring LLC
Kankakee, IL 60901 USA
US License No. 1767
CSL Behring
| ALBUMINAR-20
albumin (human) solution |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - CSL Behring LLC (058268293) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| CSL Behring LLC | 058268293 | MANUFACTURE | |