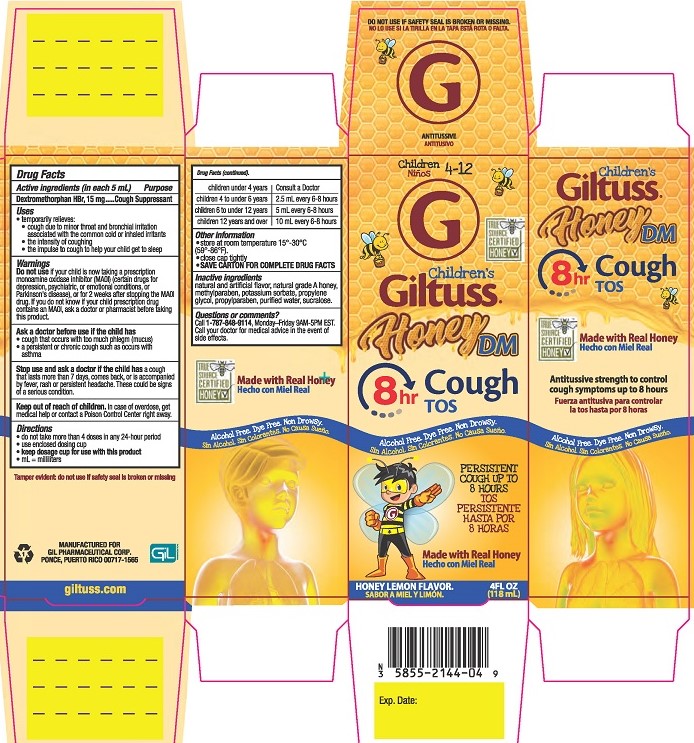
Label: CHILDRENS GILTUSS HONEY DM COUGH- dextromethorphan hbr solution
- NDC Code(s): 58552-144-04
- Packager: Gil Pharmaceutical Corp
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 24, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings
Do not useif your child is now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your child prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if the child has
- cough that occurs with too much phlegm (mucus)
- a persistent or chronic cough such as occurs with asthma
-
DOSAGE & ADMINISTRATION
Directions
- do not take more than 4 doses in any 24-hour period
- use enclosed dosing cup
- keep dosage cup for use with this product
- ml = milliliters
children under 4 years Consult a doctor children 4 to under 6 years 2.5 mL every 6-8 hours children 6 to under 12 years 5 mL every 6-8 hours children 12 years and over 10 mL every 6-8 hours - STORAGE AND HANDLING
- INACTIVE INGREDIENT
- QUESTIONS
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CHILDRENS GILTUSS HONEY DM COUGH
dextromethorphan hbr solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58552-144 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 15 mg in 5 mL Inactive Ingredients Ingredient Name Strength POTASSIUM SORBATE (UNII: 1VPU26JZZ4) WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SUCRALOSE (UNII: 96K6UQ3ZD4) HONEY (UNII: Y9H1V576FH) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) Product Characteristics Color yellow (Light yellow) Score Shape Size Flavor HONEY, LEMON Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58552-144-04 1 in 1 CARTON 06/22/2020 1 118 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 06/22/2020 Labeler - Gil Pharmaceutical Corp (176826592) Establishment Name Address ID/FEI Business Operations Dextrum Laboratories Inc 007392322 manufacture(58552-144)