Label: PANTOPRAZOLE SODIUM tablet, delayed release
-
Contains inactivated NDC Code(s)
NDC Code(s): 21695-771-30 - Packager: Rebel Distributors Corp
- This is a repackaged label.
- Source NDC Code(s): 0093-0012
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated January 3, 2011
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use pantoprazole sodium delayed-release tablets USP safely and effectively. See full prescribing information for pantoprazole sodium delayed-release tablets USP.
PANTOPRAZOLE sodium delayed-release tablets USP for oral use
Initial U.S. Approval: 2000RECENT MAJOR CHANGES
INDICATIONS AND USAGE
Pantoprazole sodium delayed-release tablets are a proton pump inhibitor indicated for the following:
DOSAGE AND ADMINISTRATION
Indication Dose Frequency Short-Term Treatment of Erosive Esophagitis Associated With GERD (2.1) Adults 40 mg Once Daily for up to 8 wks Maintenance of Healing of Erosive Esophagitis (2.1) Adults 40 mg Once Daily Pathological Hypersecretory Conditions Including Zollinger-Ellison Syndrome (2.1) Adults 40 mg Twice Daily See full prescribing information for administration instructions DOSAGE FORMS AND STRENGTHS
- Delayed-Release Tablets, 20 mg and 40 mg (3)
CONTRAINDICATIONS
Known hypersensitivity to any component of the formulation or to substituted benzimidazoles (4)
WARNINGS AND PRECAUTIONS
- Symptomatic response does not preclude presence of gastric malignancy (5.1)
- Atrophic gastritis has been noted with long-term therapy (5.2)
-
Bone Fracture
Long-term and multiple daily dose PPI therapy may be associated with an increased risk for osteoporosis-related fractures of the hip, wrist or spine. (5)
ADVERSE REACTIONS
The most frequently occurring adverse reactions are as follows:
- For adult use (> 2%) are headache, diarrhea, nausea, abdominal pain, vomiting, flatulence, dizziness, and arthralgia. (6)
To report SUSPECTED ADVERSE REACTIONS, contact TEVA USA, PHARMACOVIGILANCE at 1-888-838-2872, X6351 or drug.safety@tevausa.com; or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Do not coadminister with atazanavir or nelfinavir (7.1)
- Concomitant warfarin use may require monitoring (7.2)
- May interfere with the absorption of drugs where gastric pH is important for bioavailability (7.3)
- May produce false-positive urine screen for THC (7.4)
Information describing use in pediatric patients with erosive esophagitis associated with GERD is approved for Wyeth Pharmaceuticals Inc.’s pantoprazole sodium delayed-release tablets. However, due to Wyeth Pharmaceuticals Inc.’s marketing exclusivity rights, this drug product is not labeled with that pediatric information.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 10/2010
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Short-Term Treatment of Erosive Esophagitis Associated With Gastroesophageal Reflux Disease (GERD)
1.2 Maintenance of Healing of Erosive Esophagitis
1.3 Pathological Hypersecretory Conditions Including Zollinger-Ellison Syndrome
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosing Schedule
2.2 Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Concurrent Gastric Malignancy
5.2 Atrophic Gastritis
5.3 Cyanocobalamin (Vitamin B-12) Deficiency
5.4 Bone Fracture
5.5 Tumorigenicity
5.6 Interference With Urine Screen for THC
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Interference With Antiretroviral Therapy
7.2 Coumarin Anticoagulants
7.3 Drugs for Which Gastric pH Can Affect Bioavailability
7.4 False Positive Urine Tests for THC
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Gender
8.7 Patients With Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.4 Pharmacogenomics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Erosive Esophagitis (EE) Associated With Gastroesophageal Reflux Disease (GERD)
14.2 Long-Term Maintenance of Healing of Erosive Esophagitis
14.3 Pathological Hypersecretory Conditions Including Zollinger-Ellison Syndrome
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
Patient Counseling
FDA-Approved Patient Labeling
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
Pantoprazole sodium delayed-release tablets are indicated for:
1.1 Short-Term Treatment of Erosive Esophagitis Associated With Gastroesophageal Reflux Disease (GERD)
Pantoprazole sodium delayed-release tablets are indicated in adults for the short-term treatment (up to 8 weeks) in the healing and symptomatic relief of erosive esophagitis. For those adult patients who have not healed after 8 weeks of treatment, an additional 8 week course of pantoprazole sodium delayed-release tablets may be considered. Safety of treatment beyond 8 weeks in pediatric patients has not been established.
Pediatric indication and usage information in pediatric patients ages five years and older with erosive esophagitis associated with GERD is approved for Wyeth Pharmaceuticals Inc.’s pantoprazole sodium delayed-release tablets. However, due to Wyeth Pharmaceuticals Inc.’s marketing exclusivity rights, this drug product is not labeled with that pediatric information.
1.2 Maintenance of Healing of Erosive Esophagitis
Pantoprazole sodium delayed-release tablets are indicated for maintenance of healing of erosive esophagitis and reduction in relapse rates of daytime and nighttime heartburn symptoms in adult patients with GERD. Controlled studies did not extend beyond 12 months.
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosing Schedule
Pantoprazole sodium is supplied as delayed-release tablets. The recommended dosages are outlined in Table 1.
Table 1: Recommended Dosing Schedule for Pantoprazole Sodium Delayed-Release Tablets USP - *
- For adult patients who have not healed after 8 weeks of treatment, an additional 8 week course of pantoprazole sodium delayed-release tablets USP may be considered.
- †
- Dosage regimens should be adjusted to individual patient needs and should continue for as long as clinically indicated. Doses up to 240 mg daily have been administered.
Indication Dose Frequency Short-Term Treatment of Erosive Esophagitis Associated With GERD Adults 40 mg Once daily for up to 8 weeks* Maintenance of Healing of Erosive Esophagitis Adults 40 mg Once daily Pathological Hypersecretory Conditions Including Zollinger-Ellison Syndrome Adults 40 mg Twice daily† Pediatric dosing information in pediatric patients ages five years and older with erosive esophagitis associated with GERD is approved for Wyeth Pharmaceuticals Inc.’s pantoprazole sodium delayed-release tablets. However, due to Wyeth Pharmaceuticals Inc.’s marketing exclusivity rights, this drug product is not labeled with that pediatric information.
2.2 Administration Instructions
Directions for method of administration are presented in Table 2.
Table 2: Administration Instructions - *
- Patients should be cautioned that pantoprazole sodium delayed-release tablets USP should not be split, chewed, or crushed.
Formulation Route Instructions* Delayed-Release Tablets Oral Swallowed whole, with or without food Pantoprazole Sodium Delayed-Release Tablets USP
Pantoprazole sodium delayed-release tablets USP should be swallowed whole, with or without food in the stomach. If patients are unable to swallow a 40 mg tablet, two 20 mg tablets may be taken. Concomitant administration of antacids does not affect the absorption of pantoprazole sodium delayed-release tablets USP.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
- Pantoprazole sodium delayed-release tablets are contraindicated in patients with known hypersensitivity to any component of the formulation [see Description (11)] or any substituted benzimidazole.
-
5 WARNINGS AND PRECAUTIONS
5.1 Concurrent Gastric Malignancy
Symptomatic response to therapy with pantoprazole does not preclude the presence of gastric malignancy.
5.2 Atrophic Gastritis
Atrophic gastritis has been noted occasionally in gastric corpus biopsies from patients treated long-term with pantoprazole, particularly in patients who were H. pylori positive.
5.3 Cyanocobalamin (Vitamin B-12) Deficiency
Generally, daily treatment with any acid-suppressing medications over a long period of time (e.g., longer than 3 years) may lead to malabsorption of cyanocobalamin (Vitamin B-12) caused by hypo- or achlorhydria. Rare reports of cyanocobalamin deficiency occurring with acid-suppressing therapy have been reported in the literature. This diagnosis should be considered if clinical symptoms consistent with cyanocobalamin deficiency are observed.
5.4 Bone Fracture
Several published observational studies suggest that proton pump inhibitor (PPI) therapy may be associated with an increased risk for osteoporosis-related fractures of the hip, wrist, or spine. The risk of fracture was increased in patients who received high-dose, defined as multiple daily doses, and long-term PPI therapy (a year or longer). Patients should use the lowest dose and shortest duration of PPI therapy appropriate to the condition being treated. Patients at risk for osteoporosis-related fractures should be managed according to established treatment guidelines [see Dosage and Administration (2) and Adverse Reactions (6.2)].
5.5 Tumorigenicity
Due to the chronic nature of GERD, there may be a potential for prolonged administration of pantoprazole. In long-term rodent studies, pantoprazole was carcinogenic and caused rare types of gastrointestinal tumors. The relevance of these findings to tumor development in humans is unknown [see Nonclinical Toxicology (13.1)].
-
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Adults
Safety in nine randomized comparative U.S. clinical trials in patients with GERD included 1,473 patients on oral pantoprazole (20 mg or 40 mg), 299 patients on an H2-receptor antagonist, 46 patients on another proton pump inhibitor, and 82 patients on placebo. The most frequently occurring adverse reactions are listed in Table 3.
Table 3: Adverse Reactions Reported in Clinical Trials of Adult Patients With GERD at a Frequency of > 2% Pantoprazole Comparators Placebo (n = 1473) (n = 345) (n = 82) % % % Headache 12.2 12.8 8.5 Diarrhea 8.8 9.6 4.9 Nausea 7.0 5.2 9.8 Abdominal pain 6.2 4.1 6.1 Vomiting 4.3 3.5 2.4 Flatulence 3.9 2.9 3.7 Dizziness 3.0 2.9 1.2 Arthralgia 2.8 1.4 1.2 Additional adverse reactions that were reported for pantoprazole in clinical trials with a frequency of ≤ 2% are listed below by body system:
Body as a Whole: allergic reaction, pyrexia, photosensitivity reaction, facial edema
Gastrointestinal: constipation, dry mouth, hepatitis
Hematologic: leukopenia, thrombocytopenia
Metabolic/Nutritional: elevated CK (creatine kinase), generalized edema, elevated triglycerides, liver enzymes elevated
Musculoskeletal: myalgia
Nervous: depression, vertigo
Skin and Appendages: urticaria, rash, pruritus
Special Senses: blurred vision
Adverse reaction information in pediatric patients with erosive esophagitis associated with GERD is approved for Wyeth Pharmaceuticals Inc.’s pantoprazole sodium delayed-release tablets. However, due to Wyeth Pharmaceuticals Inc.’s marketing exclusivity rights, this drug product is not labeled with that pediatric information.
Zollinger-Ellison syndrome
In clinical studies of Zollinger-Ellison syndrome, adverse reactions reported in 35 patients taking pantoprazole 80 mg/day to 240 mg/day for up to 2 years were similar to those reported in adult patients with GERD.
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of pantoprazole. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
These adverse reactions are listed below by body system:
Immune System Disorders: anaphylaxis (including anaphylactic shock)
Skin and Subcutaneous Tissue Disorders: severe dermatologic reactions (some fatal), including erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis (TEN, some fatal), and angioedema (Quincke's edema)
Musculoskeletal and Connective Tissue Disorders: rhabdomyolysis, bone fracture
Renal and Urinary Disorders: interstitial nephritis
Hepatobiliary Disorders: hepatocellular damage leading to jaundice and hepatic failure
Psychiatric Disorders: hallucination, confusion
-
7 DRUG INTERACTIONS
7.1 Interference With Antiretroviral Therapy
Concomitant use of atazanavir or nelfinavir with proton pump inhibitors is not recommended. Coadministration of atazanavir or nelfinavir with proton pump inhibitors is expected to substantially decrease atazanavir or nelfinavir plasma concentrations and may result in a loss of therapeutic effect and development of drug resistance.
7.2 Coumarin Anticoagulants
There have been postmarketing reports of increased INR and prothrombin time in patients receiving proton pump inhibitors, including pantoprazole, and warfarin concomitantly. Increases in INR and prothrombin time may lead to abnormal bleeding and even death. Patients treated with proton pump inhibitors and warfarin concomitantly should be monitored for increases in INR and prothrombin time.
7.3 Drugs for Which Gastric pH Can Affect Bioavailability
Pantoprazole causes long-lasting inhibition of gastric acid secretion. Therefore, pantoprazole may interfere with absorption of drugs where gastric pH is an important determinant of their bioavailability (e.g., ketoconazole, ampicillin esters, and iron salts).
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic Effects
Pregnancy category B
Reproduction studies have been performed in rats at oral doses up to 88 times the recommended human dose and in rabbits at oral doses up to 16 times the recommended human dose and have revealed no evidence of impaired fertility or harm to the fetus due to pantoprazole. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed [see Nonclinical Toxicology (13.2)].
8.3 Nursing Mothers
Pantoprazole and its metabolites are excreted in the milk of rats. Pantoprazole excretion in human milk has been detected in a study of a single nursing mother after a single 40 mg oral dose. The clinical relevance of this finding is not known. Many drugs which are excreted in human milk have a potential for serious adverse reactions in nursing infants. Based on the potential for tumorigenicity shown for pantoprazole in rodent carcinogenicity studies, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the benefit of the drug to the mother.
8.4 Pediatric Use
The effectiveness of pantoprazole for treating symptomatic GERD in pediatric patients has not been established.
Information describing use in pediatric patients with erosive esophagitis associated with GERD is approved for Wyeth Pharmaceuticals Inc.’s pantoprazole sodium delayed-release tablets. However, due to Wyeth Pharmaceuticals Inc.’s marketing exclusivity rights, this drug product is not labeled with that pediatric information.
8.5 Geriatric Use
In short-term U.S. clinical trials, erosive esophagitis healing rates in the 107 elderly patients (≥ 65 years old) treated with pantoprazole were similar to those found in patients under the age of 65. The incidence rates of adverse reactions and laboratory abnormalities in patients aged 65 years and older were similar to those associated with patients younger than 65 years of age.
8.6 Gender
Erosive esophagitis healing rates in the 221 women treated with pantoprazole sodium delayed-release tablets in U.S. clinical trials were similar to those found in men. In the 122 women treated long-term with pantoprazole 40 mg or 20 mg, healing was maintained at a rate similar to that in men. The incidence rates of adverse reactions were also similar for men and women.
8.7 Patients With Hepatic Impairment
Doses higher than 40 mg/day have not been studied in patients with hepatic impairment [see Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
Experience in patients taking very high doses of pantoprazole (> 240 mg) is limited. Spontaneous postmarketing reports of overdose are generally within the known safety profile of pantoprazole.
Pantoprazole is not removed by hemodialysis. In case of overdosage, treatment should be symptomatic and supportive.
Single oral doses of pantoprazole at 709 mg/kg, 798 mg/kg, and 887 mg/kg were lethal to mice, rats, and dogs, respectively. The symptoms of acute toxicity were hypoactivity, ataxia, hunched sitting, limb-splay, lateral position, segregation, absence of ear reflex, and tremor.
-
11 DESCRIPTION
The active ingredient in pantoprazole sodium delayed-release tablets USP is a substituted benzimidazole, sodium 5-(difluoromethoxy)-2-[[(3,4-dimethoxy-2-pyridinyl)methyl]sulfinyl]-1H-benzimidazole sesquihydrate, a compound that inhibits gastric acid secretion. The structural formula is:
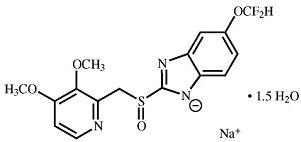
C16H14F2N3NaO4S•1.5 H2O M.W. 432.4
Pantoprazole sodium sesquihydrate is a white to off-white crystalline powder and is racemic. Pantoprazole has weakly basic and acidic properties. Pantoprazole sodium sesquihydrate is freely soluble in water, very slightly soluble in phosphate buffer at pH 7.4, and practically insoluble in n-hexane.
The stability of the compound in aqueous solution is pH-dependent. The rate of degradation increases with decreasing pH. At ambient temperature, the degradation half-life is approximately 2.8 hours at pH 5.0 and approximately 220 hours at pH 7.8.
Pantoprazole sodium is supplied as a delayed-release tablet, available in two strengths (20 mg and 40 mg).
Each pantoprazole sodium delayed-release tablet USP contains 45.1 mg or 22.6 mg of pantoprazole sodium sesquihydrate (equivalent to 40 mg or 20 mg pantoprazole, respectively) with the following inactive ingredients: calcium carbonate, calcium stearate, D&C yellow #10 aluminum lake, FD&C yellow #6 aluminum lake, hypromellose, iron oxide black, iron oxide yellow, lactose monohydrate, low-substituted hydroxypropyl cellulose, methacrylic acid copolymer, microcrystalline cellulose, polyethylene glycol, shellac glaze, sodium carbonate anhydrous, stearic acid, talc, titanium dioxide, and triethyl citrate. The imprinting ink may contain antifoam DC 1510, propylene glycol, and lecithin.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Pantoprazole is a proton pump inhibitor (PPI) that suppresses the final step in gastric acid production by covalently binding to the (H+, K+)-ATPase enzyme system at the secretory surface of the gastric parietal cell. This effect leads to inhibition of both basal and stimulated gastric acid secretion, irrespective of the stimulus. The binding to the (H+, K+)-ATPase results in a duration of antisecretory effect that persists longer than 24 hours for all doses tested (20 mg to 120 mg).
12.2 Pharmacodynamics
Antisecretory Activity
Under maximal acid stimulatory conditions using pentagastrin, a dose-dependent decrease in gastric acid output occurs after a single dose of oral (20 to 80 mg) or a single dose of intravenous (20 to 120 mg) pantoprazole in healthy volunteers. Pantoprazole given once daily results in increasing inhibition of gastric acid secretion. Following the initial oral dose of 40 mg pantoprazole, a 51% mean inhibition was achieved by 2.5 hours. With once-a-day dosing for 7 days, the mean inhibition was increased to 85%. Pantoprazole suppressed acid secretion in excess of 95% in half of the subjects. Acid secretion had returned to normal within a week after the last dose of pantoprazole; there was no evidence of rebound hypersecretion.
In a series of dose-response studies, pantoprazole, at oral doses ranging from 20 to 120 mg, caused dose-related increases in median basal gastric pH and in the percent of time gastric pH was > 3 and > 4. Treatment with 40 mg of pantoprazole produced significantly greater increases in gastric pH than the 20 mg dose. Doses higher than 40 mg (60, 80, 120 mg) did not result in further significant increases in median gastric pH. The effects of pantoprazole on median pH from one double-blind crossover study are shown in Table 4.
Table 4: Effect of Single Daily Doses of Oral Pantoprazole on Intragastric pH Median pH on day 7 Time Placebo 20 mg 40 mg 80 mg 8 a.m. to 8 a.m.
(24 hours)
1.3
2.9*
3.8*,†
3.9*,†8 a.m. to 10 p.m.
(Daytime)
1.6
3.2*
4.4*,†
4.8*,†10 p.m. to 8 a.m.
(Nighttime)
1.2
2.1*
3.0*
2.6*Serum Gastrin Effects
Fasting serum gastrin levels were assessed in two double-blind studies of the acute healing of erosive esophagitis (EE) in which 682 patients with gastroesophageal reflux disease (GERD) received 10, 20, or 40 mg of pantoprazole for up to 8 weeks. At 4 weeks of treatment there was an increase in mean gastrin levels of 7%, 35%, and 72% over pretreatment values in the 10, 20, and 40 mg treatment groups, respectively. A similar increase in serum gastrin levels was noted at the 8 week visit with mean increases of 3%, 26%, and 84% for the three pantoprazole dose groups. Median serum gastrin levels remained within normal limits during maintenance therapy with pantoprazole sodium delayed-release tablets.
In long-term international studies involving over 800 patients, a 2 to 3 fold mean increase from the pretreatment fasting serum gastrin level was observed in the initial months of treatment with pantoprazole at doses of 40 mg per day during GERD maintenance studies and 40 mg or higher per day in patients with refractory GERD. Fasting serum gastrin levels generally remained at approximately 2 to 3 times baseline for up to 4 years of periodic follow-up in clinical trials.
Following short-term treatment with pantoprazole, elevated gastrin levels return to normal by at least 3 months.
Enterochromaffin-Like (ECL) Cell Effects
In 39 patients treated with oral pantoprazole 40 mg to 240 mg daily (majority receiving 40 mg to 80 mg) for up to 5 years, there was a moderate increase in ECL-cell density, starting after the first year of use, which appeared to plateau after 4 years.
In a nonclinical study in Sprague-Dawley rats, lifetime exposure (24 months) to pantoprazole at doses of 0.5 to 200 mg/kg/day resulted in dose-related increases in gastric ECL cell proliferation and gastric neuroendocrine (NE)-cell tumors. Gastric NE-cell tumors in rats may result from chronic elevation of serum gastrin concentrations. The high density of ECL cells in the rat stomach makes this species highly susceptible to the proliferative effects of elevated gastrin concentrations produced by proton pump inhibitors. However, there were no observed elevations in serum gastrin following the administration of pantoprazole at a dose of 0.5 mg/kg/day. In a separate study, a gastric NE-cell tumor without concomitant ECL-cell proliferative changes was observed in 1 female rat following 12 months of dosing with pantoprazole at 5 mg/kg/day and a 9 month off-dose recovery [see Nonclinical Toxicology (13.1)].
12.3 Pharmacokinetics
Pantoprazole sodium delayed-release tablets are prepared as enteric-coated tablets so that absorption of pantoprazole begins only after the tablet leaves the stomach. Peak serum concentration (Cmax) and area under the serum concentration time curve (AUC) increase in a manner proportional to oral and intravenous doses from 10 mg to 80 mg. Pantoprazole does not accumulate, and its pharmacokinetics are unaltered with multiple daily dosing. Following oral or intravenous administration, the serum concentration of pantoprazole declines biexponentially, with a terminal elimination half-life of approximately one hour.
In extensive metabolizers with normal liver function receiving an oral dose of the enteric-coated 40 mg pantoprazole tablet, the peak concentration (Cmax) is 2.5 mcg/mL; the time to reach the peak concentration (tmax) is 2.5 h, and the mean total area under the plasma concentration versus time curve (AUC) is 4.8 mcg•h/mL (range 1.4 to 13.3 mcg•h/mL). Following intravenous administration of pantoprazole to extensive metabolizers, its total clearance is 7.6 to 14.0 L/h, and its apparent volume of distribution is 11.0 to 23.6 L.
Absorption
After administration of a single or multiple oral 40 mg doses of pantoprazole sodium delayed-release tablets, the peak plasma concentration of pantoprazole was achieved in approximately 2.5 hours, and Cmax was 2.5 mcg/mL. Pantoprazole undergoes little first-pass metabolism, resulting in an absolute bioavailability of approximately 77%. Pantoprazole absorption is not affected by concomitant administration of antacids.
Administration of pantoprazole sodium delayed-release tablets with food may delay its absorption up to 2 hours or longer; however, the Cmax and the extent of pantoprazole absorption (AUC) are not altered. Thus, pantoprazole sodium delayed-release tablets may be taken without regard to timing of meals.
Distribution
The apparent volume of distribution of pantoprazole is approximately 11.0 to 23.6 L, distributing mainly in extracellular fluid. The serum protein binding of pantoprazole is about 98%, primarily to albumin.
Metabolism
Pantoprazole is extensively metabolized in the liver through the cytochrome P450 (CYP) system. Pantoprazole metabolism is independent of the route of administration (intravenous or oral). The main metabolic pathway is demethylation, by CYP2C19, with subsequent sulfation; other metabolic pathways include oxidation by CYP3A4. There is no evidence that any of the pantoprazole metabolites have significant pharmacologic activity.
Elimination
After a single oral or intravenous dose of 14C-labeled pantoprazole to healthy, normal metabolizer volunteers, approximately 71% of the dose was excreted in the urine, with 18% excreted in the feces through biliary excretion. There was no renal excretion of unchanged pantoprazole.
Geriatric
Only slight to moderate increases in pantoprazole AUC (43%) and Cmax (26%) were found in elderly volunteers (64 to 76 years of age) after repeated oral administration, compared with younger subjects. No dosage adjustment is recommended based on age.
Pediatric
Pharmacokinetic information in pediatric patients is approved for Wyeth Pharmaceuticals Inc.’s pantoprazole sodium delayed-release tablets. However, due to Wyeth Pharmaceuticals Inc.’s marketing exclusivity rights, this drug product is not labeled with that pediatric information.
Gender
There is a modest increase in pantoprazole AUC and Cmax in women compared to men. However, weight-normalized clearance values are similar in women and men. No dosage adjustment is recommended based on gender. In pediatric patients ages 1 through 16 years there were no clinically relevant effects of gender on clearance of pantoprazole, as shown by population pharmacokinetic analysis.
Renal Impairment
In patients with severe renal impairment, pharmacokinetic parameters for pantoprazole were similar to those of healthy subjects. No dosage adjustment is necessary in patients with renal impairment or in patients undergoing hemodialysis.
Hepatic Impairment
In patients with mild to severe hepatic impairment (Child-Pugh A to C cirrhosis), maximum pantoprazole concentrations increased only slightly (1.5 fold) relative to healthy subjects. Although serum half-life values increased to 7 to 9 hours and AUC values increased by 5 to 7 fold in hepatic-impaired patients, these increases were no greater than those observed in CYP2C19 poor metabolizers, where no dosage adjustment is warranted. These pharmacokinetic changes in hepatic-impaired patients result in minimal drug accumulation following once-daily, multiple-dose administration. No dosage adjustment is needed in patients with mild to severe hepatic impairment. Doses higher than 40 mg/day have not been studied in hepatically impaired patients.
Drug-Drug Interactions
Pantoprazole is metabolized mainly by CYP2C19 and to minor extents by CYPs 3A4, 2D6, and 2C9. In in vivo drug-drug interaction studies with CYP2C19 substrates (diazepam [also a CYP3A4 substrate] and phenytoin [also a CYP3A4 inducer]), nifedipine, midazolam, and clarithromycin (CYP3A4 substrates), metoprolol (a CYP2D6 substrate), diclofenac, naproxen and piroxicam (CYP2C9 substrates), and theophylline (a CYP1A2 substrate) in healthy subjects, the pharmacokinetics of pantoprazole were not significantly altered.
In vivo studies also suggest that pantoprazole does not significantly affect the kinetics of the following drugs (cisapride, theophylline, diazepam [and its active metabolite, desmethyldiazepam], phenytoin, warfarin, metoprolol, nifedipine, carbamazepine, midazolam, clarithromycin, naproxen, piroxicam, and oral contraceptives [levonorgestrel/ethinyl estradiol]). Dosage adjustment of these drugs is not necessary when they are coadministered with pantoprazole. In other in vivo studies, digoxin, ethanol, glyburide, antipyrine, caffeine, metronidazole, and amoxicillin had no clinically relevant interactions with pantoprazole.
Based on studies evaluating possible interactions of pantoprazole with other drugs, no dosage adjustment is needed with concomitant use of the following: theophylline, cisapride, antipyrine, caffeine, carbamazepine, diazepam (and its active metabolite, desmethyldiazepam), diclofenac, naproxen, piroxicam, digoxin, ethanol, glyburide, an oral contraceptive (levonorgestrel/ethinyl estradiol), metoprolol, nifedipine, phenytoin, warfarin, midazolam, clarithromycin, metronidazole, or amoxicillin.
There was also no interaction with concomitantly administered antacids.
There have been postmarketing reports of increased INR and prothrombin time in patients receiving proton pump inhibitors, including pantoprazole, and warfarin concomitantly [see Drug Interactions (7.2)].
Although no significant drug-drug interactions have been observed in clinical studies, the potential for significant drug-drug interactions with more than once-daily dosing with high doses of pantoprazole has not been studied in poor metabolizers or individuals who are hepatically impaired.
Other Effects
In a clinical pharmacology study, pantoprazole 40 mg given once daily for 2 weeks had no effect on the levels of the following hormones: cortisol, testosterone, triiodothyronine (T3), thyroxine (T4), thyroid-stimulating hormone (TSH), thyronine-binding protein, parathyroid hormone, insulin, glucagon, renin, aldosterone, follicle-stimulating hormone, luteinizing hormone, prolactin, and growth hormone.
In a 1 year study of GERD patients treated with pantoprazole 40 mg or 20 mg, there were no changes from baseline in overall levels of T3, T4, and TSH.
12.4 Pharmacogenomics
CYP2C19 displays a known genetic polymorphism due to its deficiency in some subpopulations (e.g., approximately 3% of Caucasians and African-Americans and 17% to 23% of Asians are poor metabolizers). Although these subpopulations of pantoprazole poor metabolizers have elimination half-life values of 3.5 to 10.0 hours in adults, they still have minimal accumulation (≤ 23%) with once-daily dosing. For adult patients who are CYP2C19 poor metabolizers, no dosage adjustment is needed.
Similar to adults, pediatric patients who have the poor metabolizer genotype of CYP2C19 (CYP2C19 *2/*2) exhibited greater than a 6 fold increase in AUC compared to pediatric extensive (CYP2C19 *1/*1) and intermediate (CYP2C19 *1/*x) metabolizers. Poor metabolizers exhibited approximately 10 fold lower apparent oral clearance compared to extensive metabolizers.
For known pediatric poor metabolizers, a dose reduction should be considered.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 24 month carcinogenicity study, Sprague-Dawley rats were treated orally with doses of 0.5 to 200 mg/kg/day, about 0.1 to 40 times the exposure on a body surface area basis of a 50 kg person dosed at 40 mg/day. In the gastric fundus, treatment at 0.5 to 200 mg/kg/day produced enterochromaffin-like (ECL) cell hyperplasia and benign and malignant neuroendocrine cell tumors in a dose-related manner. In the forestomach, treatment at 50 and 200 mg/kg/day (about 10 and 40 times the recommended human dose on a body surface area basis) produced benign squamous cell papillomas and malignant squamous cell carcinomas. Rare gastrointestinal tumors associated with pantoprazole treatment included an adenocarcinoma of the duodenum at 50 mg/kg/day and benign polyps and adenocarcinomas of the gastric fundus at 200 mg/kg/day. In the liver, treatment at 0.5 to 200 mg/kg/day produced dose-related increases in the incidences of hepatocellular adenomas and carcinomas. In the thyroid gland, treatment at 200 mg/kg/day produced increased incidences of follicular cell adenomas and carcinomas for both male and female rats.
In a 24 month carcinogenicity study, Fischer 344 rats were treated orally with doses of 5 to 50 mg/kg/day, approximately 1 to 10 times the recommended human dose based on body surface area. In the gastric fundus, treatment at 5 to 50 mg/kg/day produced enterochromaffin-like (ECL) cell hyperplasia and benign and malignant neuroendocrine cell tumors. Dose selection for this study may not have been adequate to comprehensively evaluate the carcinogenic potential of pantoprazole.
In a 24 month carcinogenicity study, B6C3F1 mice were treated orally with doses of 5 to 150 mg/kg/day, 0.5 to 15 times the recommended human dose based on body surface area. In the liver, treatment at 150 mg/kg/day produced increased incidences of hepatocellular adenomas and carcinomas in female mice. Treatment at 5 to 150 mg/kg/day also produced gastric-fundic ECL cell hyperplasia.
A 26 week p53 +/- transgenic mouse carcinogenicity study was not positive.
Pantoprazole was positive in the in vitro human lymphocyte chromosomal aberration assays, in one of two mouse micronucleus tests for clastogenic effects, and in the in vitro Chinese hamster ovarian cell/HGPRT forward mutation assay for mutagenic effects. Equivocal results were observed in the in vivo rat liver DNA covalent binding assay. Pantoprazole was negative in the in vitro Ames mutation assay, the in vitro unscheduled DNA synthesis (UDS) assay with rat hepatocytes, the in vitro AS52/GPT mammalian cell-forward gene mutation assay, the in vitro thymidine kinase mutation test with mouse lymphoma L5178Y cells, and the in vivo rat bone marrow cell chromosomal aberration assay.
There were no effects on fertility or reproductive performance when pantoprazole was given at oral doses up to 500 mg/kg/day in male rats (98 times the recommended human dose based on body surface area) and 450 mg/kg/day in female rats (88 times the recommended human dose based on body surface area).
13.2 Animal Toxicology and/or Pharmacology
Studies in neonatal/juvenile and adult rats and dogs were performed. The data from these studies revealed that animals in both age groups respond to pantoprazole in a similar manner. Gastric alterations, including increased stomach weights, increased incidence of eosinophilic chief cells in adult and neonatal/juvenile rats, and atrophy of chief cells in adult rats and in neonatal/juvenile dogs, were observed in the fundic mucosa of stomachs in repeated-dose studies. Decreases in red cell mass parameters, increases in cholesterol and triglycerides, increased liver weight, enzyme induction, and hepatocellular hypertrophy were also seen in repeated-dose studies in rats and/or dogs. Full to partial recovery of these effects were noted in animals of both age groups following a recovery period.
Reproductive Toxicology Studies
Reproduction studies have been performed in rats at oral doses up to 450 mg/kg/day (88 times the recommended human dose based on body surface area) and rabbits at oral doses up to 40 mg/kg/day (16 times the recommended human dose based on body surface area) and have revealed no evidence of impaired fertility or harm to the fetus due to pantoprazole.
-
14 CLINICAL STUDIES
Pantoprazole sodium delayed-release tablets were used in the following clinical trials.
14.1 Erosive Esophagitis (EE) Associated With Gastroesophageal Reflux Disease (GERD)
Adult Patients
A U.S. multicenter, double-blind, placebo-controlled study of pantoprazole 10 mg, 20 mg, or 40 mg once daily was conducted in 603 patients with reflux symptoms and endoscopically diagnosed EE of grade 2 or above (Hetzel-Dent scale). In this study, approximately 25% of enrolled patients had severe EE of grade 3, and 10% had grade 4. The percentages of patients healed (per protocol, n = 541) in this study are shown in Table 7.
Table 7: Erosive Esophagitis Healing Rates (Per Protocol) Pantoprazole Placebo
Week10 mg daily
(n = 153)20 mg daily
(n = 158)40 mg daily
(n = 162)
(n = 68)4 45.6%* 58.4%*,† 75.0%*,‡ 14.3% 8 66.0%* 83.5%*,† 92.6%*,‡ 39.7% In this study, all pantoprazole treatment groups had significantly greater healing rates than the placebo group. This was true regardless of H. pylori status for the 40 mg and 20 mg pantoprazole treatment groups. The 40 mg dose of pantoprazole resulted in healing rates significantly greater than those found with either the 20 mg or 10 mg dose.
A significantly greater proportion of patients taking pantoprazole 40 mg experienced complete relief of daytime and nighttime heartburn and the absence of regurgitation, starting from the first day of treatment, compared with placebo. Patients taking pantoprazole consumed significantly fewer antacid tablets per day than those taking placebo.
Pantoprazole 40 mg and 20 mg once daily were also compared with nizatidine 150 mg twice daily in a U.S. multicenter, double-blind study of 243 patients with reflux symptoms and endoscopically diagnosed EE of grade 2 or above. The percentages of patients healed (per protocol, n = 212) are shown in Table 8.
Table 8: Erosive Esophagitis Healing Rates (Per Protocol) - *
- (p < 0.001) pantoprazole versus nizatidine
Pantoprazole Nizatidine
Week20 mg daily
(n = 72)40 mg daily
(n = 70)150 mg twice daily
(n = 70)4 61.4%* 64.0%* 22.2% 8 79.2%* 82.9%* 41.4% Once-daily treatment with pantoprazole 40 mg or 20 mg resulted in significantly superior rates of healing at both 4 and 8 weeks compared with twice-daily treatment with 150 mg of nizatidine. For the 40 mg treatment group, significantly greater healing rates compared to nizatidine were achieved regardless of the H. pylori status.
A significantly greater proportion of the patients in the pantoprazole treatment groups experienced complete relief of nighttime heartburn and regurgitation, starting on the first day and of daytime heartburn on the second day, compared with those taking nizatidine 150 mg twice daily. Patients taking pantoprazole consumed significantly fewer antacid tablets per day than those taking nizatidine.
Clinical study information in pediatric patients ages five years through 16 years with erosive esophagitis associated with GERD is approved for Wyeth Pharmaceuticals Inc.’s pantoprazole sodium delayed-release tablets. However, due to Wyeth Pharmaceuticals Inc.’s marketing exclusivity rights, this drug product is not labeled with that pediatric information.
14.2 Long-Term Maintenance of Healing of Erosive Esophagitis
Two independent, multicenter, randomized, double-blind, comparator-controlled trials of identical design were conducted in adult GERD patients with endoscopically confirmed healed erosive esophagitis to demonstrate efficacy of pantoprazole in long-term maintenance of healing. The two U.S. studies enrolled 386 and 404 patients, respectively, to receive either 10 mg, 20 mg, or 40 mg of pantoprazole sodium delayed-release tablets once daily or 150 mg of ranitidine twice daily. As demonstrated in Table 9, pantoprazole 40 mg and 20 mg were significantly superior to ranitidine at every timepoint with respect to the maintenance of healing. In addition, pantoprazole 40 mg was superior to all other treatments studied.
Table 9: Long-Term Maintenance of Healing of Erosive Gastroesophageal Reflux Disease (GERD Maintenance): Percentage of Patients Who Remained Healed Pantoprazole
20 mg dailyPantoprazole
40 mg dailyRanitidine
150 mg twice dailyStudy 1 n = 75 n = 74 n = 75 Month 1 91* 99* 68 Month 3 82* 93*,† 54 Month 6 76* 90*,† 44 Month 12 70* 86*,† 35 Study 2 n = 74 n = 88 n = 84 Month 1 89* 92*,† 62 Month 3 78* 91*,† 47 Month 6 72* 88*,† 39 Month 12 72* 83* 37 Note: Pantoprazole 10 mg was superior (p < 0.05) to ranitidine in Study 2, but not Study 1.
Pantoprazole 40 mg was superior to ranitidine in reducing the number of daytime and nighttime heartburn episodes from the first through the twelfth month of treatment. Pantoprazole 20 mg, administered once daily, was also effective in reducing episodes of daytime and nighttime heartburn in one trial, as presented in Table 10.
Table 10: Number of Episodes of Heartburn (Mean ± SD) - *
- (p < 0.001 vs. ranitidine, combined data from the two U.S. studies)
Pantoprazole Ranitidine 40 mg daily 150 mg twice daily Month 1 Daytime 5.1 ± 1.6* 18.3 ± 1.6 Nighttime 3.9 ± 1.1* 11.9 ± 1.1 Month 12 Daytime 2.9 ± 1.5* 17.5 ± 1.5 Nighttime 2.5 ± 1.2* 13.8 ± 1.3 14.3 Pathological Hypersecretory Conditions Including Zollinger-Ellison Syndrome
In a multicenter, open-label trial of 35 patients with pathological hypersecretory conditions, such as Zollinger-Ellison syndrome, with or without multiple endocrine neoplasia-type I, pantoprazole successfully controlled gastric acid secretion. Doses ranging from 80 mg daily to 240 mg daily maintained gastric acid output below 10 mEq/h in patients without prior acid-reducing surgery and below 5 mEq/h in patients with prior acid-reducing surgery.
Doses were initially titrated to the individual patient needs, and adjusted in some patients based on the clinical response with time [see Dosage and Administration (2)]. Pantoprazole was well tolerated at these dose levels for prolonged periods (greater than 2 years in some patients).
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
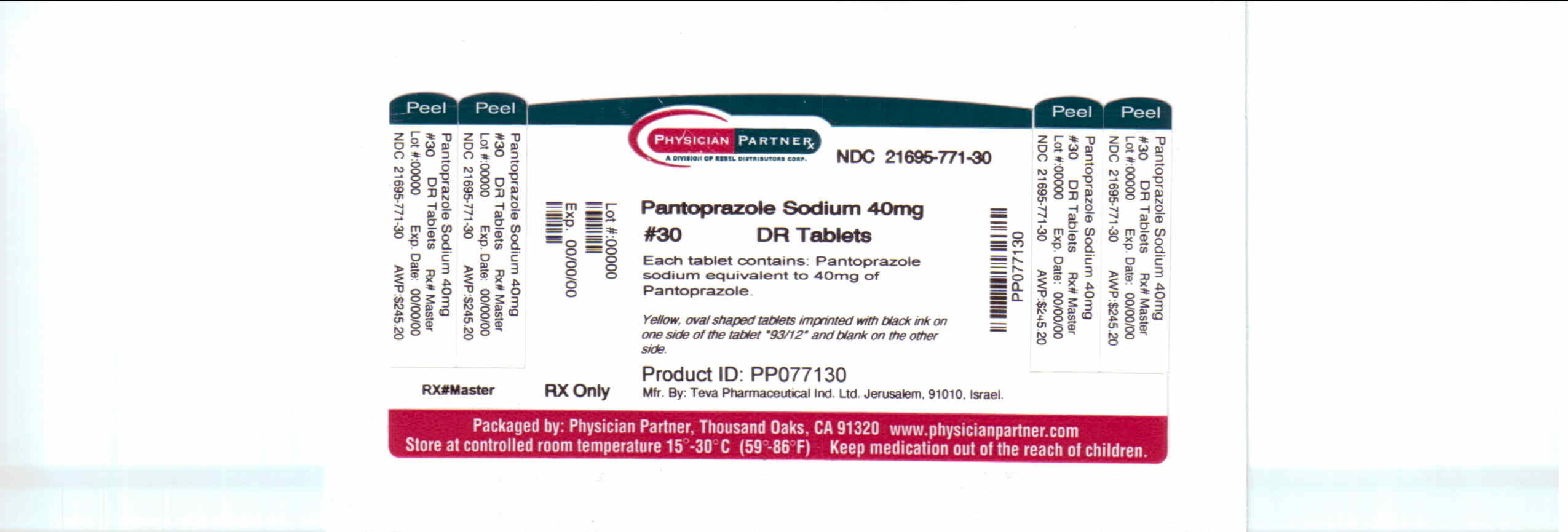
Pantoprazole sodium NDC 21695-771-30 delayed-release tablets USP are available as:
40 mg - yellow, oval shaped tablets imprinted with black ink on one side of the tablet “93/12” and blank on the other side. They are available in bottles of 30.
Storage
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
Dispense in a tight, light-resistant container as defined in the USP, with a child-resistant closure (as required).
-
17 PATIENT COUNSELING INFORMATION
See FDA-Approved Patient Labeling.
Patient Counseling
- Caution patients that pantoprazole sodium delayed-release tablets should not be split, crushed, or chewed.
- Tell patients that pantoprazole sodium delayed-release tablets should be swallowed whole, with or without food in the stomach.
- Let patients know that concomitant administration of antacids does not affect the absorption of pantoprazole sodium delayed-release tablets.
Manufactured In Israel By:
TEVA PHARMACEUTICAL IND. LTD.
Jerusalem, 91010, Israel
Manufactured For:
TEVA PHARMACEUTICALS USA
Sellersville, PA 18960
Rev. D 9/2010
FDA-Approved Patient Labeling
PATIENT INFORMATION
Pantoprazole Sodium Delayed-Release Tablets USP
Read the Patient Information that comes with pantoprazole sodium delayed-release tablets USP before you start taking them and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your doctor about your medical condition or your treatment.
What are pantoprazole sodium delayed-release tablets USP?
Pantoprazole sodium delayed-release tablets USP are a prescription medicine called a proton pump inhibitor (PPI).
Pantoprazole sodium delayed-release tablets USP are used in adults for:
- Up to 8 weeks for short-term treatment of acid-related damage to the lining of the esophagus (erosive esophagitis) caused by gastroesophageal reflux disease (GERD). If needed, your doctor may prescribe an additional 8 weeks of pantoprazole sodium delayed-release tablets USP
- Maintain healing of acid-related damage to the lining of the esophagus and helps prevent return of heartburn symptoms caused by GERD. Pantoprazole sodium delayed-release tablets USP have not been studied for treatment lasting longer than 1 year
- Treating a rare condition called Zollinger-Ellison syndrome, where the stomach makes more than the normal amount of acid
Information describing use in pediatric patients ages five years through 16 years old with erosive esophagitis associated with GERD is approved for Wyeth Pharmaceuticals Inc.’s pantoprazole sodium delayed-release tablets. However, due to Wyeth Pharmaceuticals Inc.’s marketing exclusivity rights, this drug product is not labeled with that pediatric information.
Pantoprazole sodium delayed-release tablets USP are not for children under 5 years old.
Who should not take pantoprazole sodium delayed-release tablets USP?
Do not take pantoprazole sodium delayed-release tablets USP if you are:
- allergic to any of the ingredients in pantoprazole sodium delayed-release tablets USP. See the end of this leaflet for a complete list of ingredients in pantoprazole sodium delayed-release tablets USP.
- allergic to any proton pump inhibitor (PPI). If you do not know if your medicines are PPIs, please ask your doctor.
What should I tell my doctor before taking pantoprazole sodium delayed-release tablets USP?
Before taking pantoprazole sodium delayed-release tablets USP, tell your doctor about all your medical conditions, including if you are:
- pregnant, think you may be pregnant, or are planning to become pregnant. It is not known if pantoprazole sodium delayed-release tablets USP will harm your unborn baby. Talk to your doctor if you are pregnant or plan to become pregnant.
- breastfeeding or planning to breastfeed. Pantoprazole may pass into your milk. Talk with your doctor about the best way to feed your baby if you take pantoprazole sodium delayed-release tablets USP.
Tell your doctor about all of the medicines you take, including prescription and non-prescription drugs, vitamins and herbal supplements. Pantoprazole sodium delayed-release tablets USP may affect how other medicines work, and other medicines may affect how pantoprazole sodium delayed-release tablets USP work. Especially tell your doctor if you take:
- Warfarin (Coumadin®, Athrombin-K™, Jantoven®, Panwarfin®)
- Ketoconazole (Nizoral®)
- Atazanavir (Reyataz®), Nelfinavir (Viracept®)
- Iron supplements
- Ampicillin antibiotics
Ask your doctor if you are not sure if any of your medicines are the kind listed above.
How should I take pantoprazole sodium delayed-release tablets USP?
- Take pantoprazole sodium delayed-release tablets USP exactly as prescribed by your doctor.
- Do not change your dose or stop pantoprazole sodium delayed-release tablets USP without talking to your doctor.
- If you forget to take a dose of pantoprazole sodium delayed-release tablets USP, take it as soon as you remember. If it is almost time for your next dose, do not take the missed dose. Take the next dose at your regular time. Do not take two doses to try to make up for a missed dose.
- If you take too many pantoprazole sodium delayed-release tablets USP, call your doctor right away.
- See the Patient Instructions for Use at the end of this leaflet for detailed instructions about how to take pantoprazole sodium delayed-release tablets USP.
What are the possible side effects of pantoprazole sodium delayed-release tablets USP?
Pantoprazole sodium delayed-release tablets USP can cause serious side effects including:
- Stomach lining weakening with long-term use
- Vitamin B-12 deficiency
- Serious allergic reactions. Tell your doctor if you get any of the following symptoms with pantoprazole sodium delayed-release tablets USP
- rash
- face swelling
- throat tightness
- difficult breathing
Your doctor may stop pantoprazole sodium delayed-release tablets USP if these symptoms happen.
The most common side effects with pantoprazole sodium delayed-release tablets USP in adults include:
- Headache
- Diarrhea
- Nausea
- Stomach pain
- Vomiting
- Gas
- Dizziness
- Pain in your joints
The most common side effects with pantoprazole sodium delayed-release tablets USP in children include:
- Upper respiratory infection
- Headache
- Fever
- Diarrhea
- Vomiting
- Rash
- Stomach pain
People who are taking multiple daily doses of proton pump inhibitor medicines for a long period of time may have an increased risk of fractures of the hip, wrist or spine.
Tell your doctor about any side effects that bother you or that do not go away.
These are not all the possible side effects with pantoprazole sodium delayed-release tablets USP. Talk with your doctor or pharmacist if you have any questions about side effects. Call your doctor for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088.
How should I store pantoprazole sodium delayed-release tablets USP?
- Store pantoprazole sodium delayed-release tablets USP at room temperature between 20° to 25°C (68° to 77°F).
- Keep pantoprazole sodium delayed-release tablets USP and all medicines out of the reach of children.
General Information
Medicines are sometimes prescribed for purposes other than those listed in the Patient Information leaflet. Do not use pantoprazole sodium delayed-release tablets USP for a condition for which they were not prescribed. Do not give pantoprazole sodium delayed-release tablets USP to other people, even if they have the same symptoms you have. They may harm them.
This Patient Information leaflet provides a summary of the most important information about pantoprazole sodium delayed-release tablets USP. For more information, ask your doctor. You can ask your doctor or pharmacist for information that is written for healthcare professionals.
For more information, call 1-888-838-2872, MEDICAL AFFAIRS.
What are the ingredients in pantoprazole sodium delayed-release tablets USP?
Active ingredient: pantoprazole sodium sesquihydrate
Inactive ingredients in pantoprazole sodium delayed-release tablets USP: calcium carbonate, calcium stearate, D&C yellow #10 aluminum lake, FD&C yellow #6 aluminum lake, hypromellose, iron oxide black, iron oxide yellow, lactose monohydrate, low-substituted hydroxypropyl cellulose, methacrylic acid copolymer, microcrystalline cellulose, polyethylene glycol, shellac glaze, sodium carbonate anhydrous, stearic acid, talc, titanium dioxide, and triethyl citrate. The imprinting ink may contain antifoam DC 1510, propylene glycol, and lecithin.
Patient Instructions for Use
- You can take pantoprazole sodium delayed-release tablets USP with food or on an empty stomach.
- Swallow pantoprazole sodium delayed-release tablets USP whole.
- If you have trouble swallowing a pantoprazole sodium delayed-release tablet USP, 40 mg, you can take two 20 mg tablets instead.
- Do not split, chew, or crush pantoprazole sodium delayed-release tablets USP.
All brand names listed are the registered trademarks of their respective owners and are not trademarks of Teva Pharmaceuticals USA.
Manufactured In Israel By:
TEVA PHARMACEUTICAL IND. LTD.
Jerusalem, 91010, Israel
Manufactured For:
TEVA PHARMACEUTICALS USA
Sellersville, PA 18960
Rev. A 9/2010
Repackaged by:
REBEL DISTRIBUTORS CORP
Thousand Oaks, CA 91320
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PANTOPRAZOLE SODIUM
pantoprazole sodium tablet, delayed releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:21695-771(NDC:0093-0012) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PANTOPRAZOLE SODIUM (UNII: 6871619Q5X) (PANTOPRAZOLE - UNII:D8TST4O562) PANTOPRAZOLE 40 mg Inactive Ingredients Ingredient Name Strength CALCIUM CARBONATE (UNII: H0G9379FGK) CALCIUM STEARATE (UNII: 776XM7047L) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) ALUMINUM OXIDE (UNII: LMI26O6933) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) HYPROMELLOSES (UNII: 3NXW29V3WO) FERROSOFERRIC OXIDE (UNII: XM0M87F357) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) HYDROXYPROPYL CELLULOSE, LOW SUBSTITUTED (UNII: 2165RE0K14) METHACRYLIC ACID - ETHYL ACRYLATE COPOLYMER (1:1) TYPE A (UNII: NX76LV5T8J) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL (UNII: 3WJQ0SDW1A) SHELLAC (UNII: 46N107B71O) SODIUM CARBONATE (UNII: 45P3261C7T) STEARIC ACID (UNII: 4ELV7Z65AP) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) Product Characteristics Color YELLOW Score no score Shape OVAL Size 10mm Flavor Imprint Code 93;12 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:21695-771-30 30 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA077056 10/12/2010 Labeler - Rebel Distributors Corp (118802834) Establishment Name Address ID/FEI Business Operations Rebel Distributors Corp 118802834 RELABEL, REPACK