TRIPLE ANTIBIOTIC- bacitracin zinc, neomycin sulfate, and polymyxin b sulfate ointment
H2-Pharma, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Triple Antibiotic Ointment
Active ingredients (in each gram)
Bacitracin zinc USP, 400 units
Neomycin 3.5 mg
Polymyxin B sulfate USP, 5,000 units
Warnings
For external use only.
Directions
- clean the affected area
- apply a small amount of this product (an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily
- may be covered with a sterile bandage
Other information
- store at 15°-30°C (59°-86°F). Protect from freezing.
- before using any medication, read all label directions. Keep carton, it contains important information.
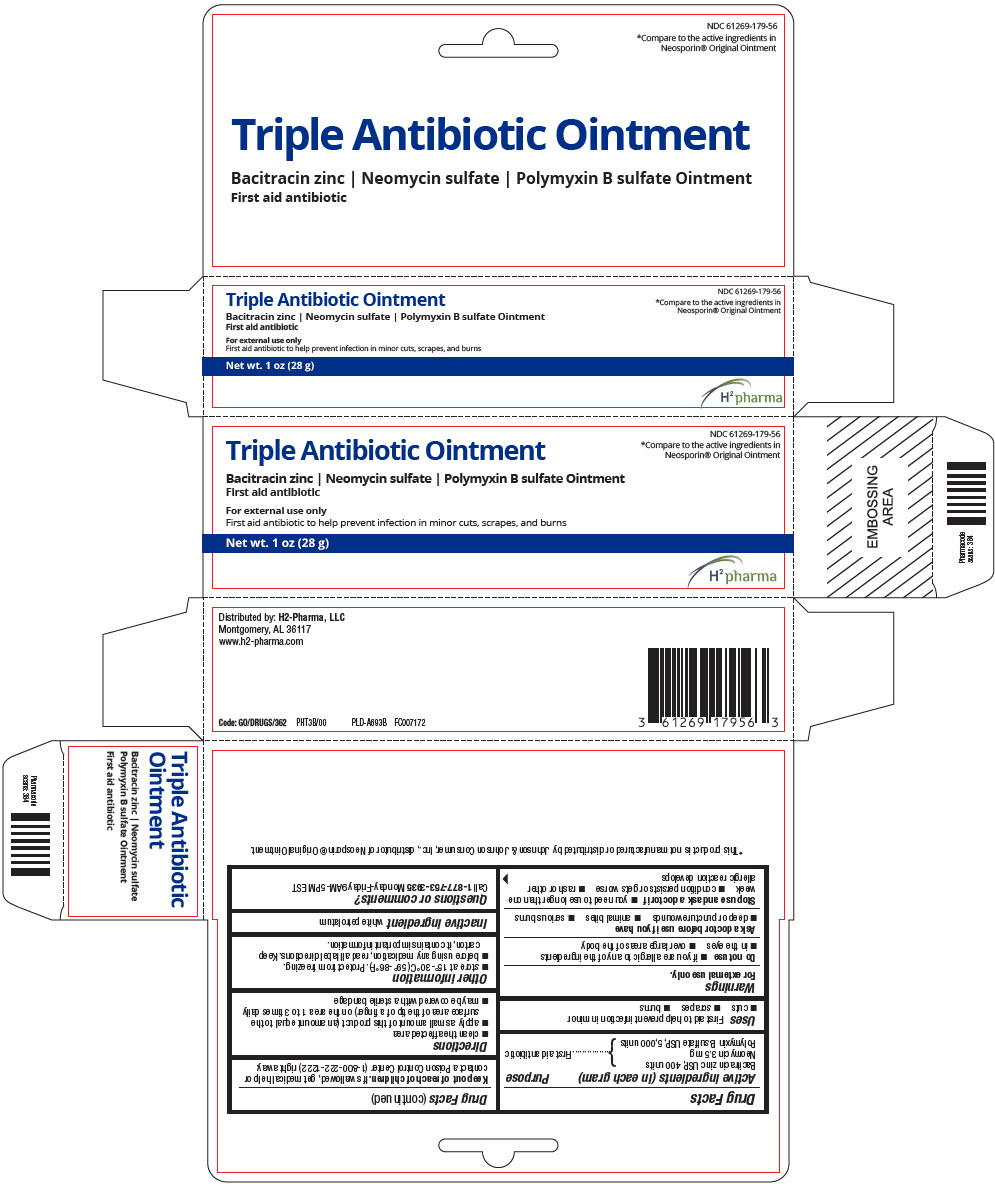
PRINCIPAL DISPLAY PANEL - 28 g Tube Carton
NDC 61269-179-56
*Compare to the active ingredients in
Neosporin® Original Ointment
Triple Antibiotic Ointment
Bacitracin zinc | Neomycin sulfate | Polymyxin B sulfate Ointment
First aid antibiotic
For external use only
First aid antibiotic to help prevent infection in minor cuts, scrapes, and burns
Net wt. 1 oz (28 g)
H2 pharma
| TRIPLE ANTIBIOTIC
bacitracin zinc, neomycin sulfate, and polymyxin b sulfate ointment |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - H2-Pharma, LLC (028473634) |
Revised: 2/2023
Document Id: 1fd0a2b4-b802-4971-89c8-1097ac7b3226
Set id: a68f5b34-591d-4a91-9f73-0bb5a37f2c5f
Version: 3
Effective Time: 20230207
H2-Pharma, LLC