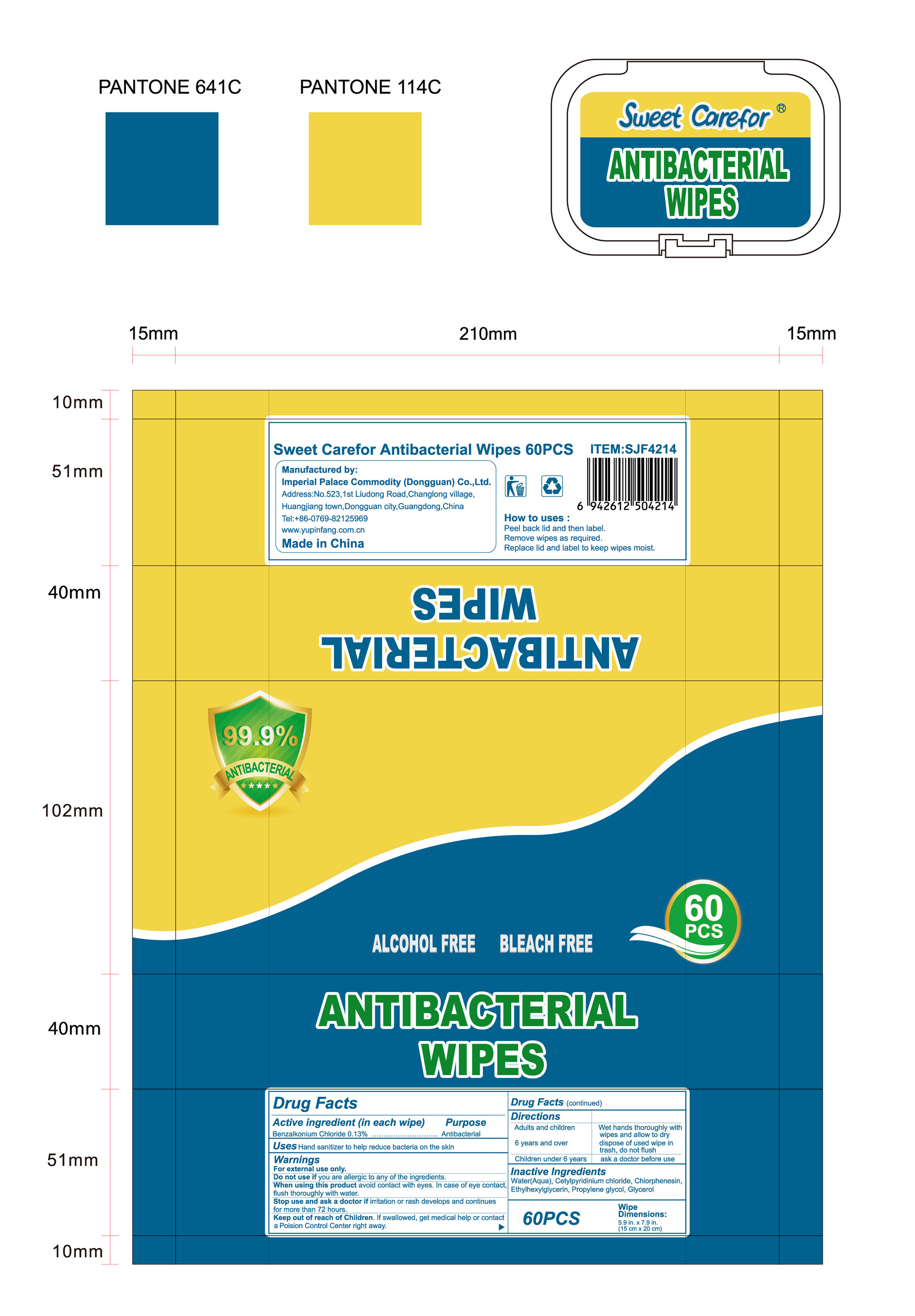
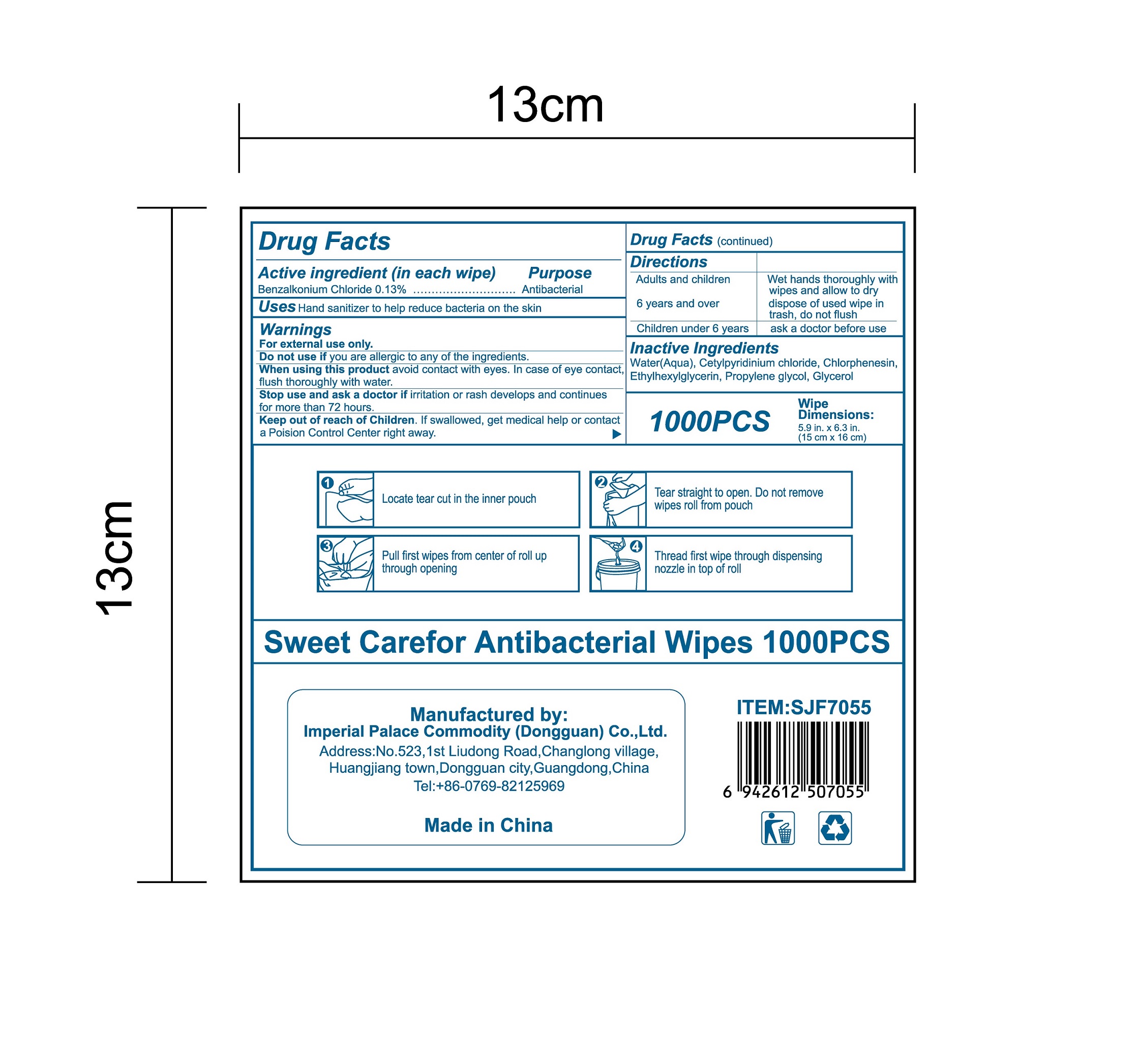
Label: ANTIBACTERIAL WIPES- benzalkonium chloride cloth
-
NDC Code(s):
52489-002-01,
52489-002-02,
52489-002-03,
52489-002-04, view more52489-002-05, 52489-002-06, 52489-002-07, 52489-002-08, 52489-002-09
- Packager: Imperial Palace Commodity (shenzhen) Co., Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 20, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient(s)
- Purpose
- Use
- Warnings
- Do not use
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- Directions
-
How to uses
Packs
●Peel back lid and then label.
●Remove wipes as required.
●Replace lid and label to keep wipes moist.
Buckets
●Open the bucket lid from the top.
●Locate tear cut in the inner pouch, tear straight to open. Do not remove wipesroll from pouch.
●Pull first wipes from center of roll up through opening.
●Thread first wipe through dispensing nozzle in top of roll.
●Close the bucket lid and dispense the wipes as required.
- Inactive ingredients
- Package Label - Principal Display Panel
-
INGREDIENTS AND APPEARANCE
ANTIBACTERIAL WIPES
benzalkonium chloride clothProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:52489-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 1.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) GLYCERIN (UNII: PDC6A3C0OX) CETYLPYRIDINIUM CHLORIDE (UNII: D9OM4SK49P) WATER (UNII: 059QF0KO0R) CHLORPHENESIN (UNII: I670DAL4SZ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52489-002-01 360 mL in 1 PACKAGE; Type 0: Not a Combination Product 05/27/2020 2 NDC:52489-002-02 370 mL in 1 PAIL; Type 0: Not a Combination Product 05/27/2020 3 NDC:52489-002-03 940 mL in 1 PAIL; Type 0: Not a Combination Product 05/27/2020 4 NDC:52489-002-04 3000 mL in 1 PAIL; Type 0: Not a Combination Product 05/27/2020 5 NDC:52489-002-05 3.3 mL in 1 PACKAGE; Type 0: Not a Combination Product 07/22/2020 6 NDC:52489-002-06 4.6 mL in 1 PACKAGE; Type 0: Not a Combination Product 07/22/2020 7 NDC:52489-002-07 230 mL in 1 PACKAGE; Type 0: Not a Combination Product 07/22/2020 8 NDC:52489-002-08 280 mL in 1 PACKAGE; Type 0: Not a Combination Product 07/22/2020 9 NDC:52489-002-09 3560 mL in 1 PACKAGE; Type 0: Not a Combination Product 09/09/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 05/27/2020 Labeler - Imperial Palace Commodity (shenzhen) Co., Ltd (527796368) Registrant - Imperial Palace Commodity(Dongguan) CO.,LTD (544367643) Establishment Name Address ID/FEI Business Operations Imperial Palace Commodity(Dongguan) CO.,LTD 544367643 manufacture(52489-002)



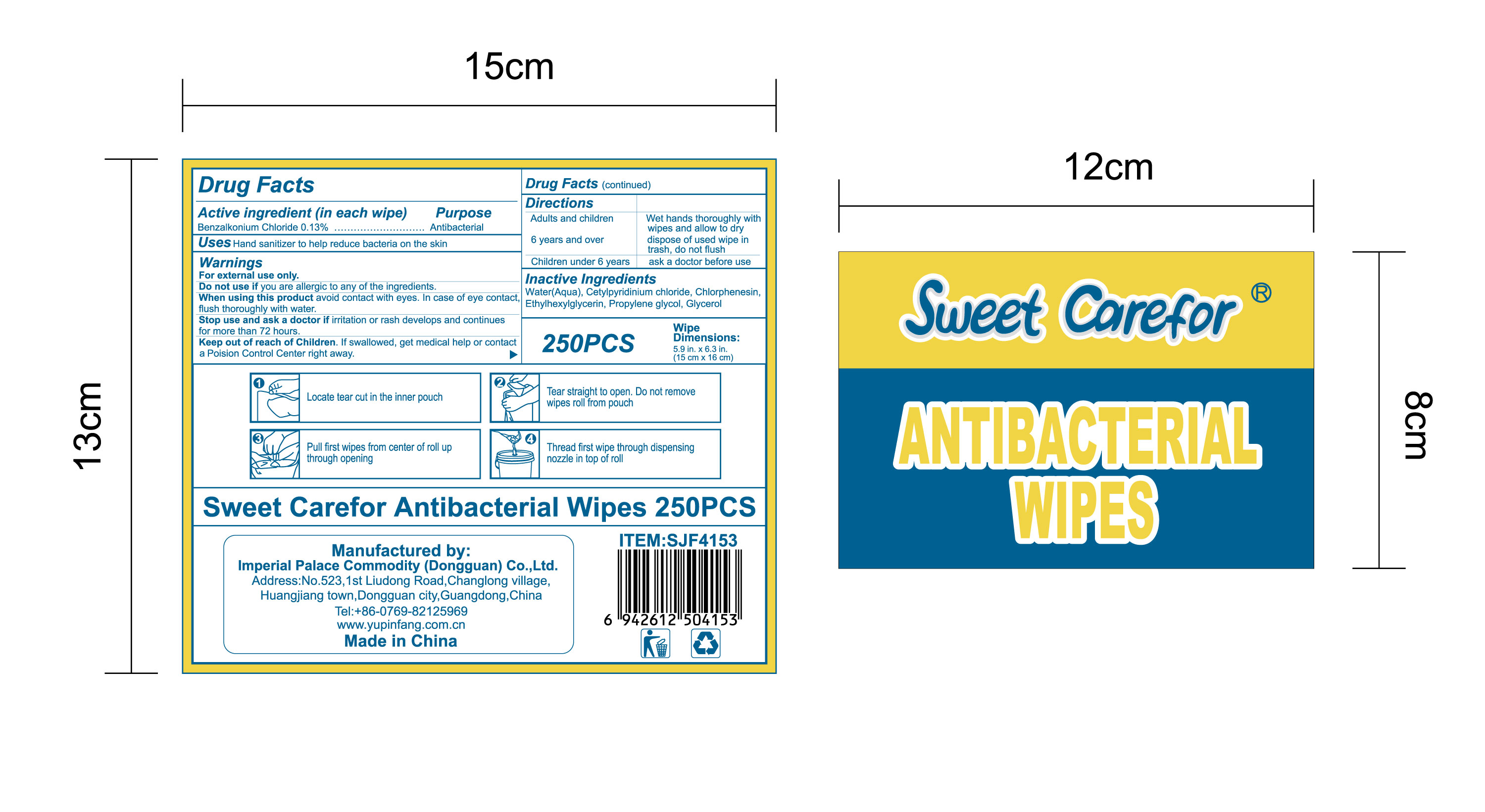


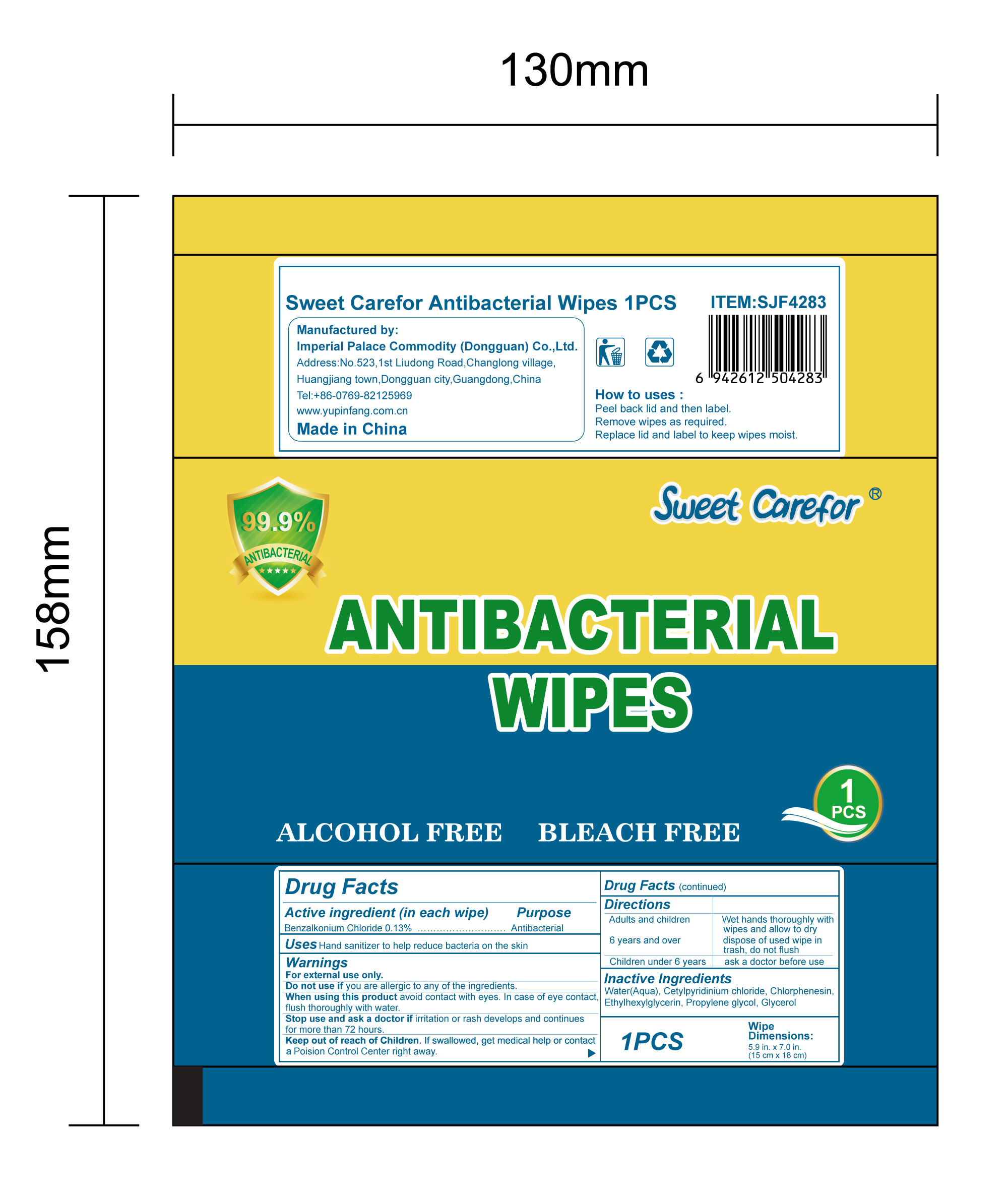
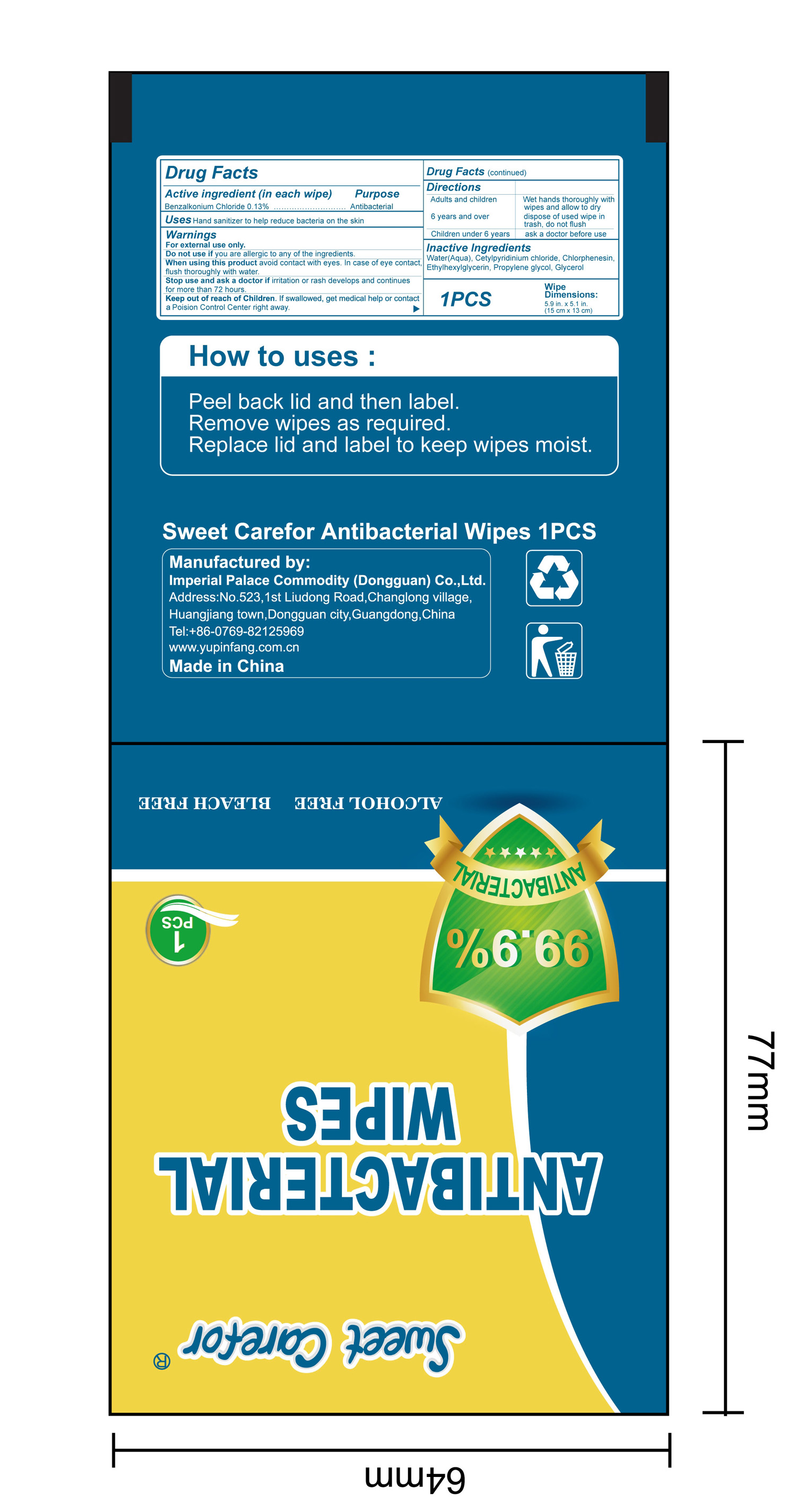

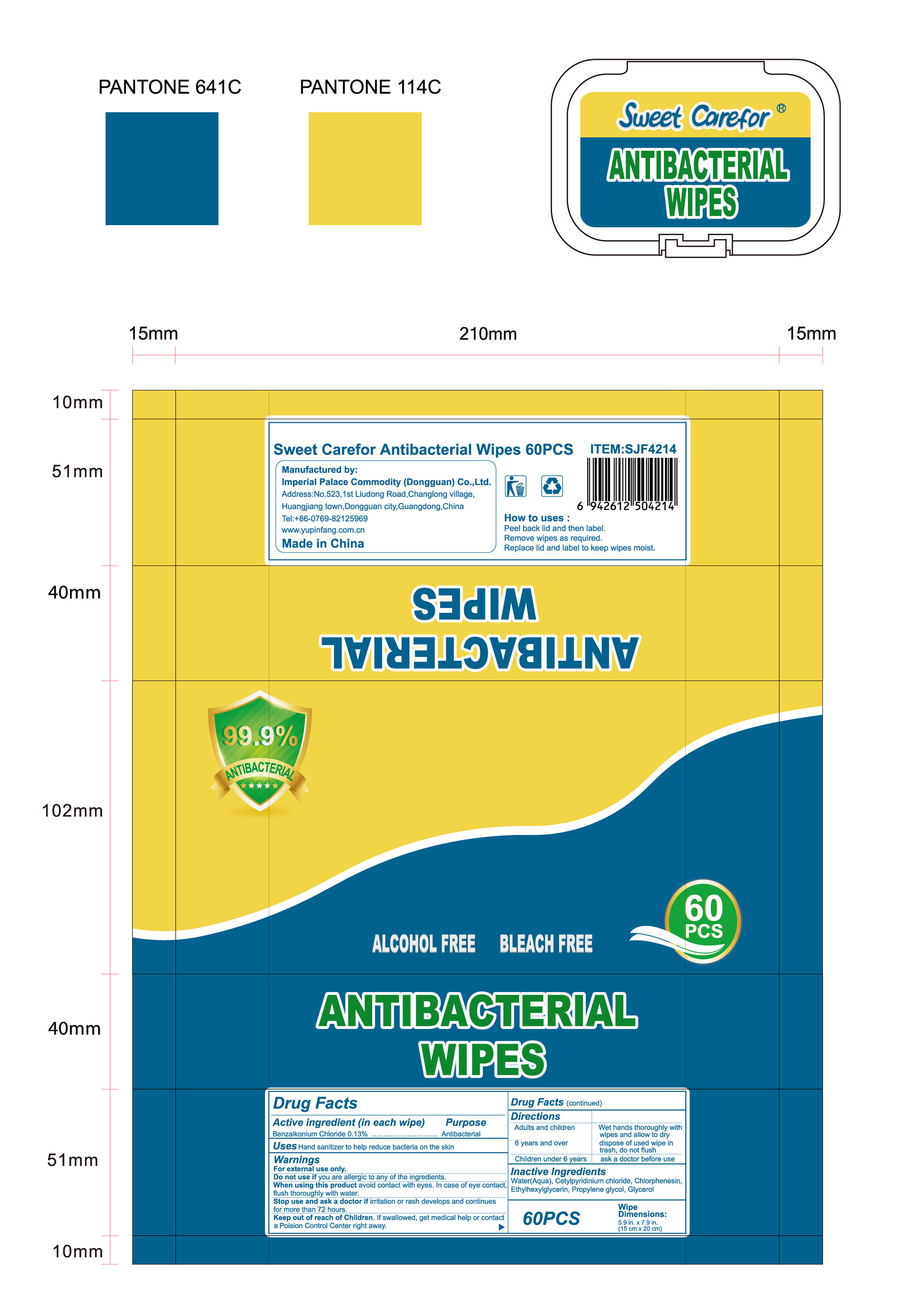
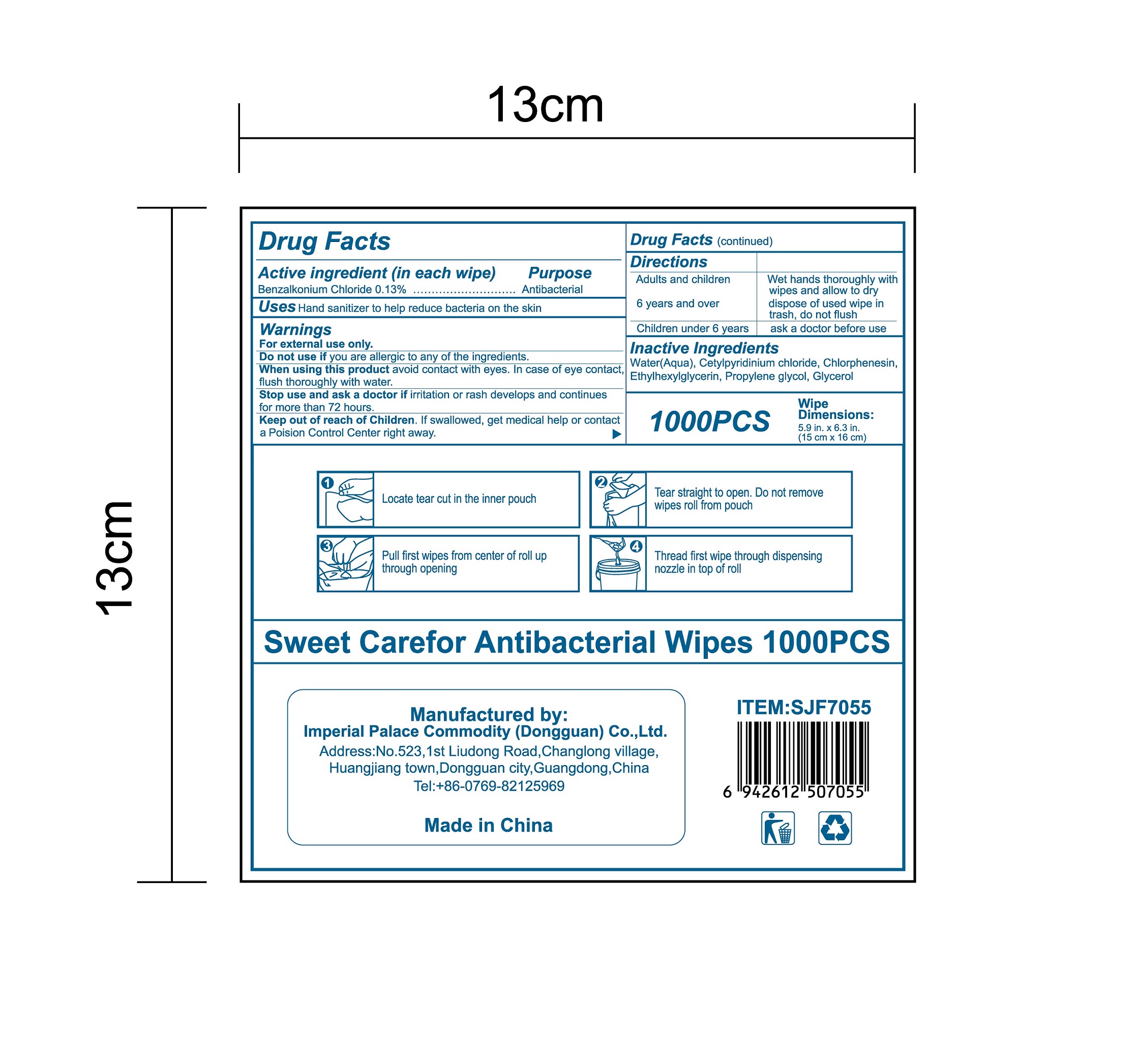
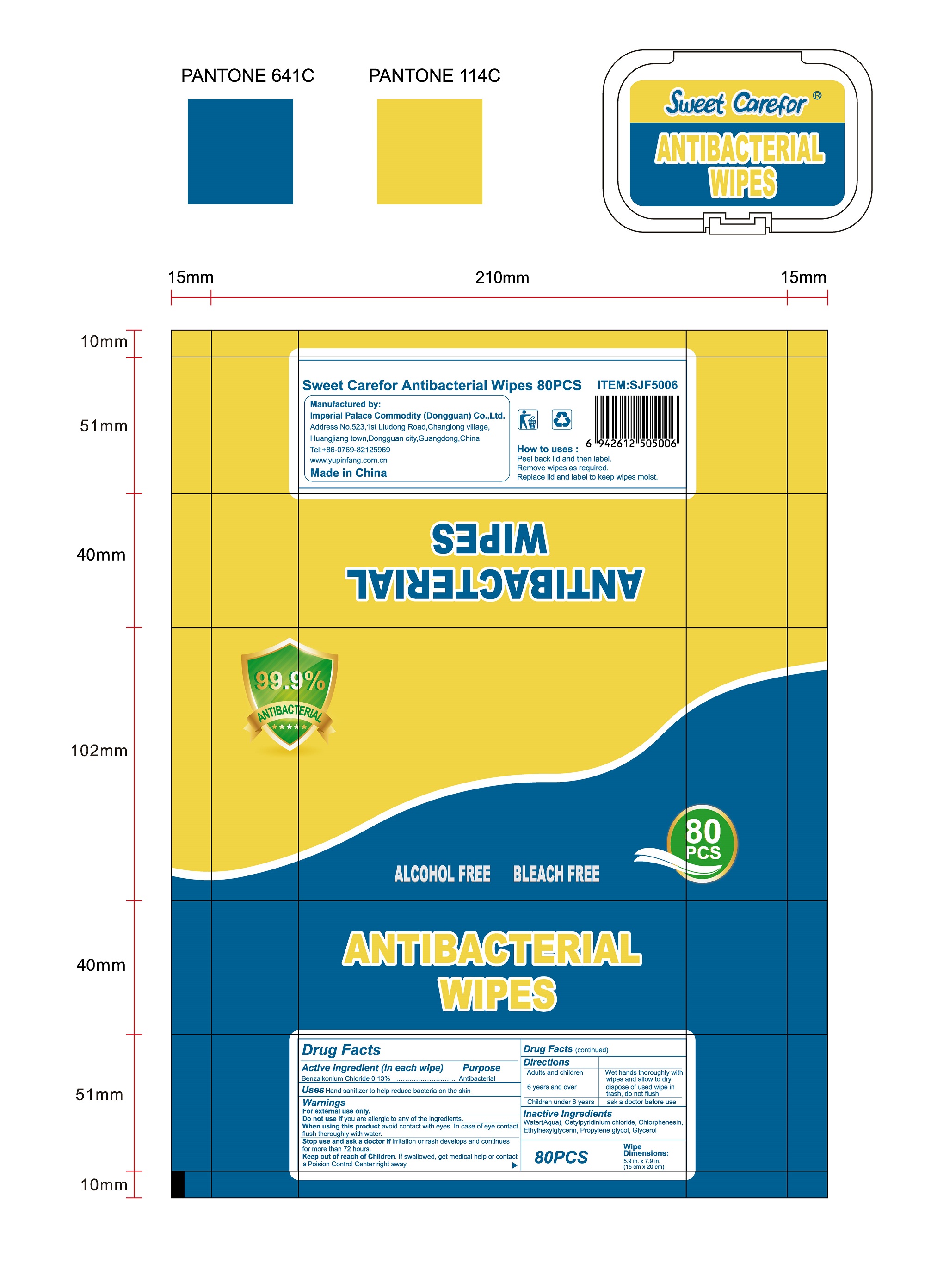

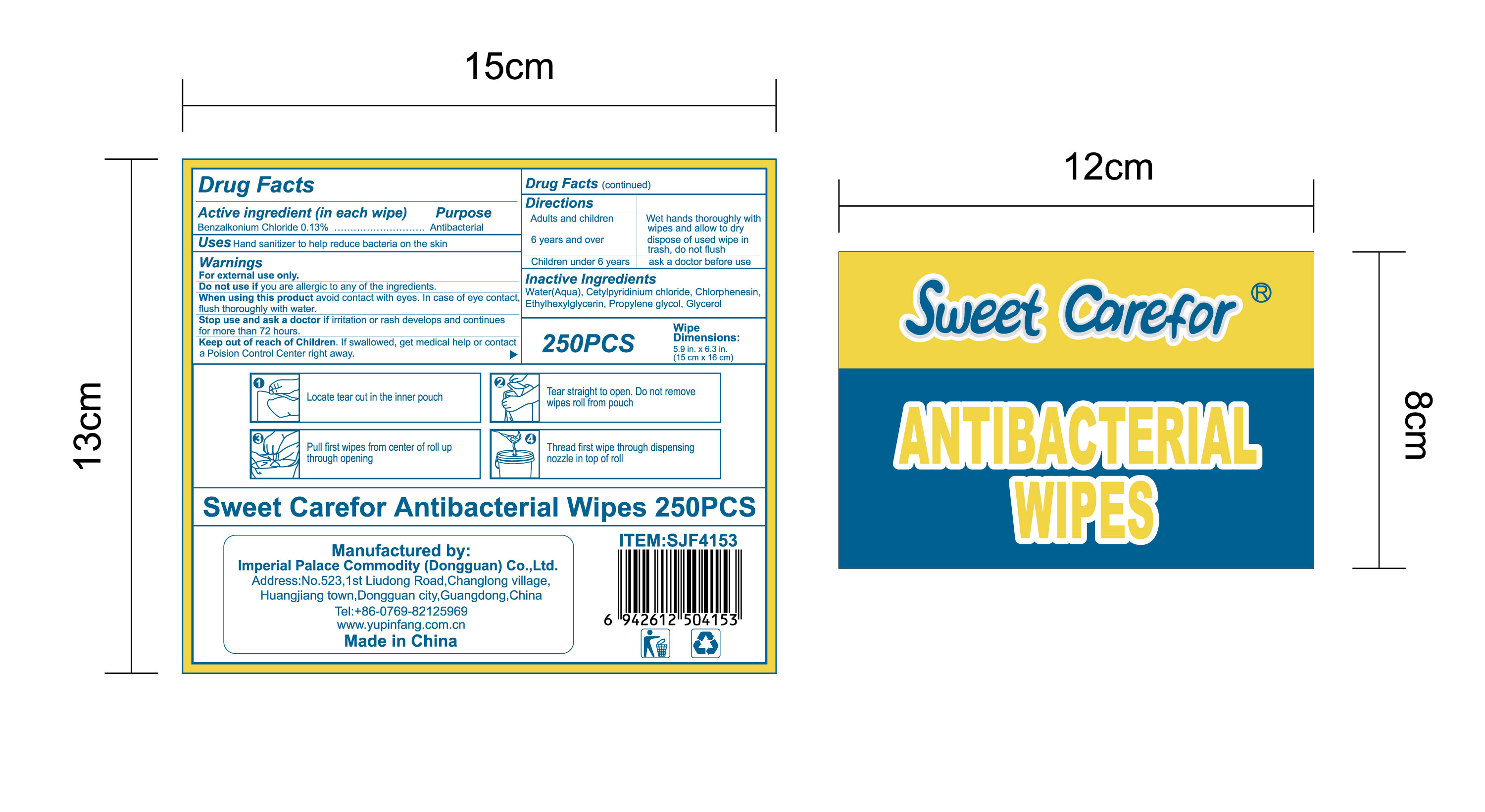

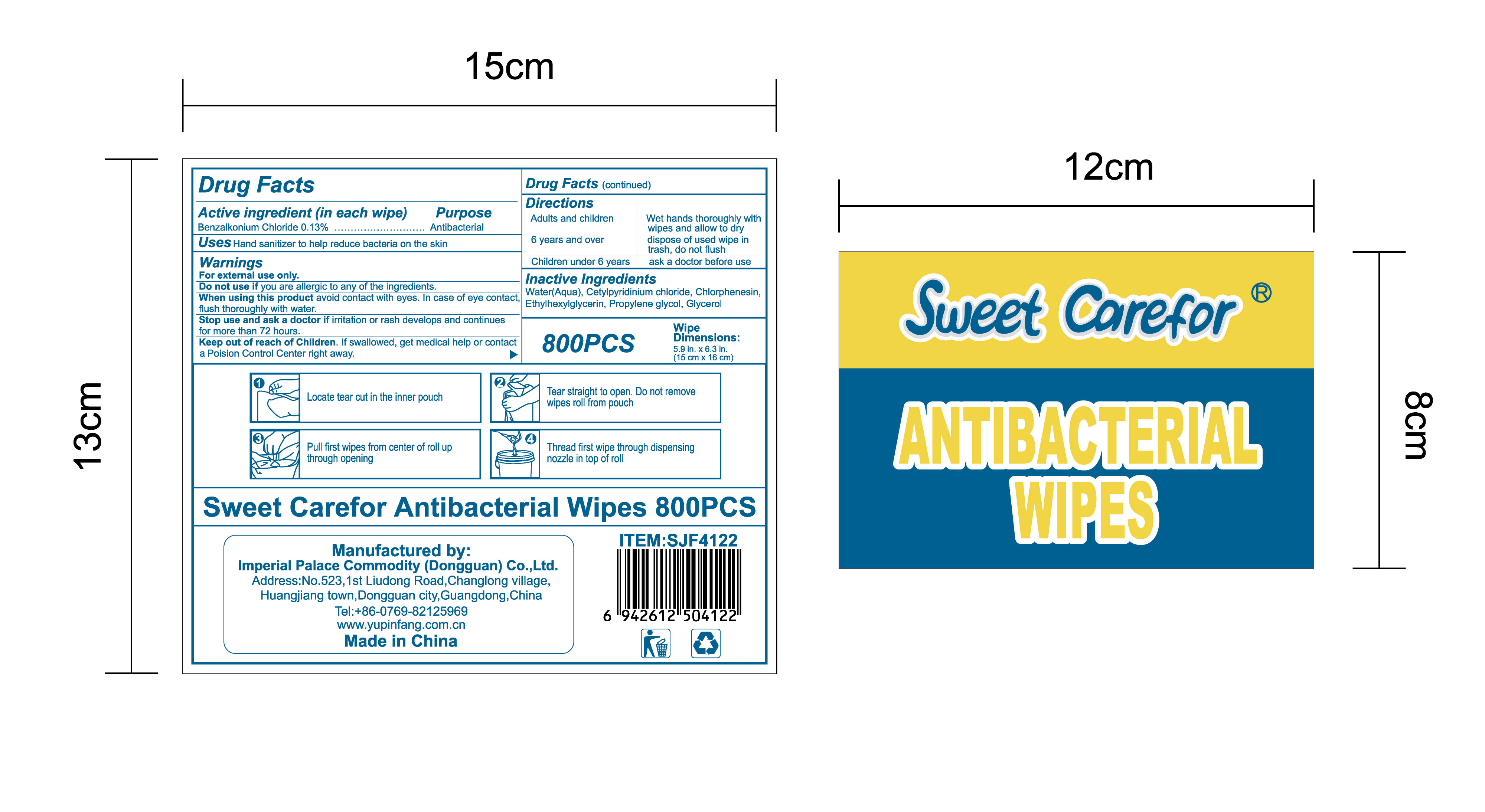

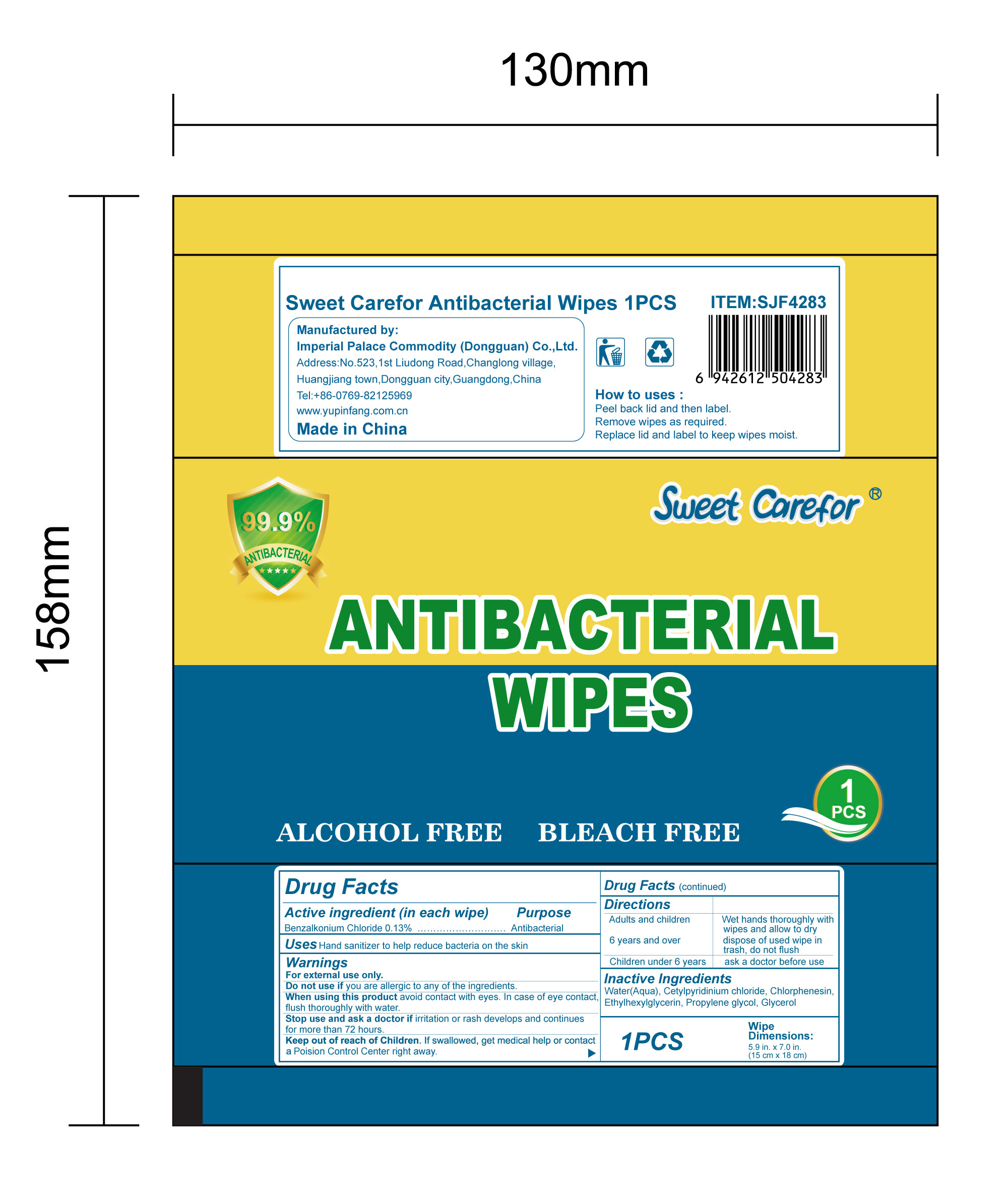 NDC:52489-002-06
NDC:52489-002-06
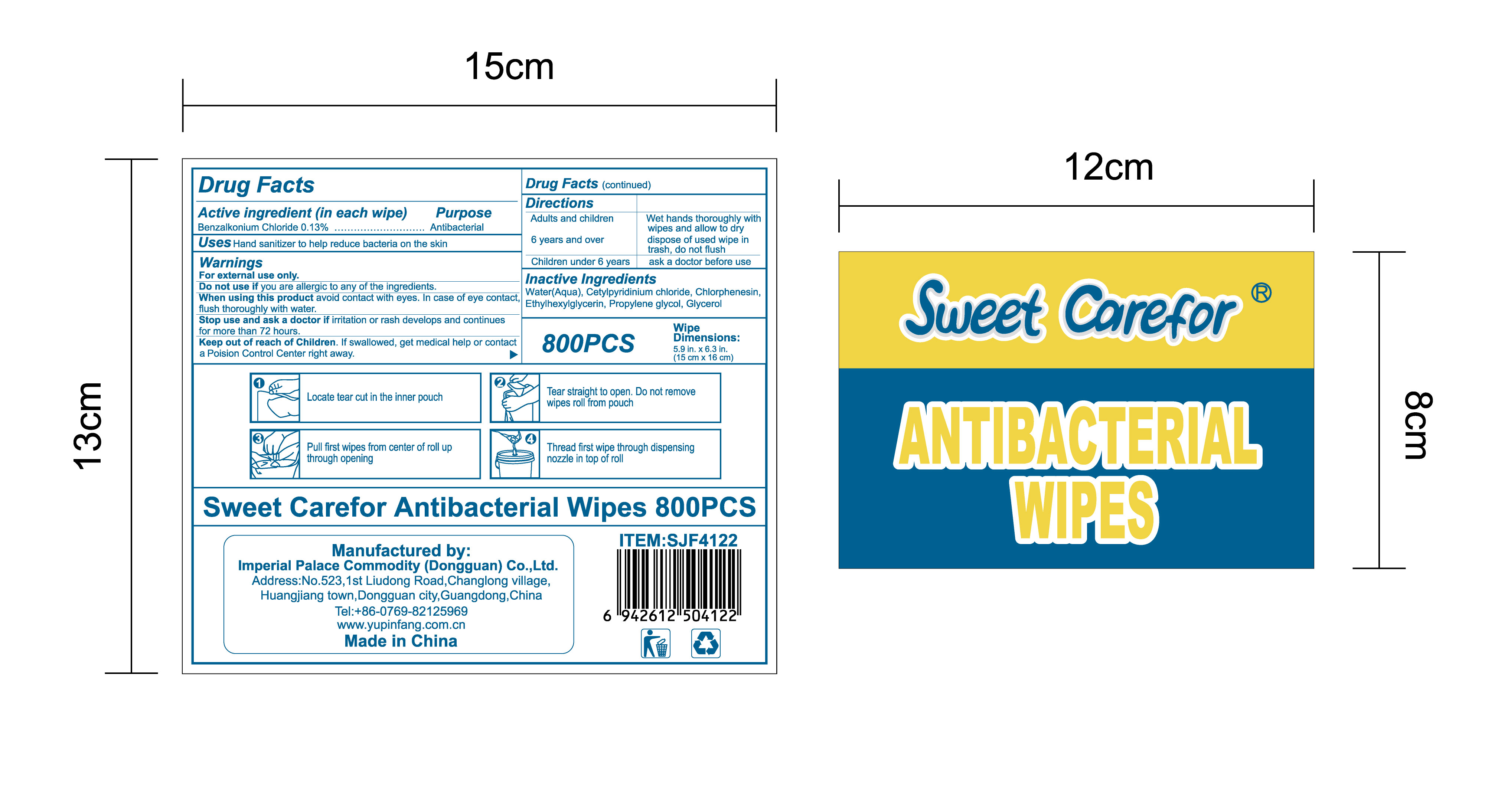
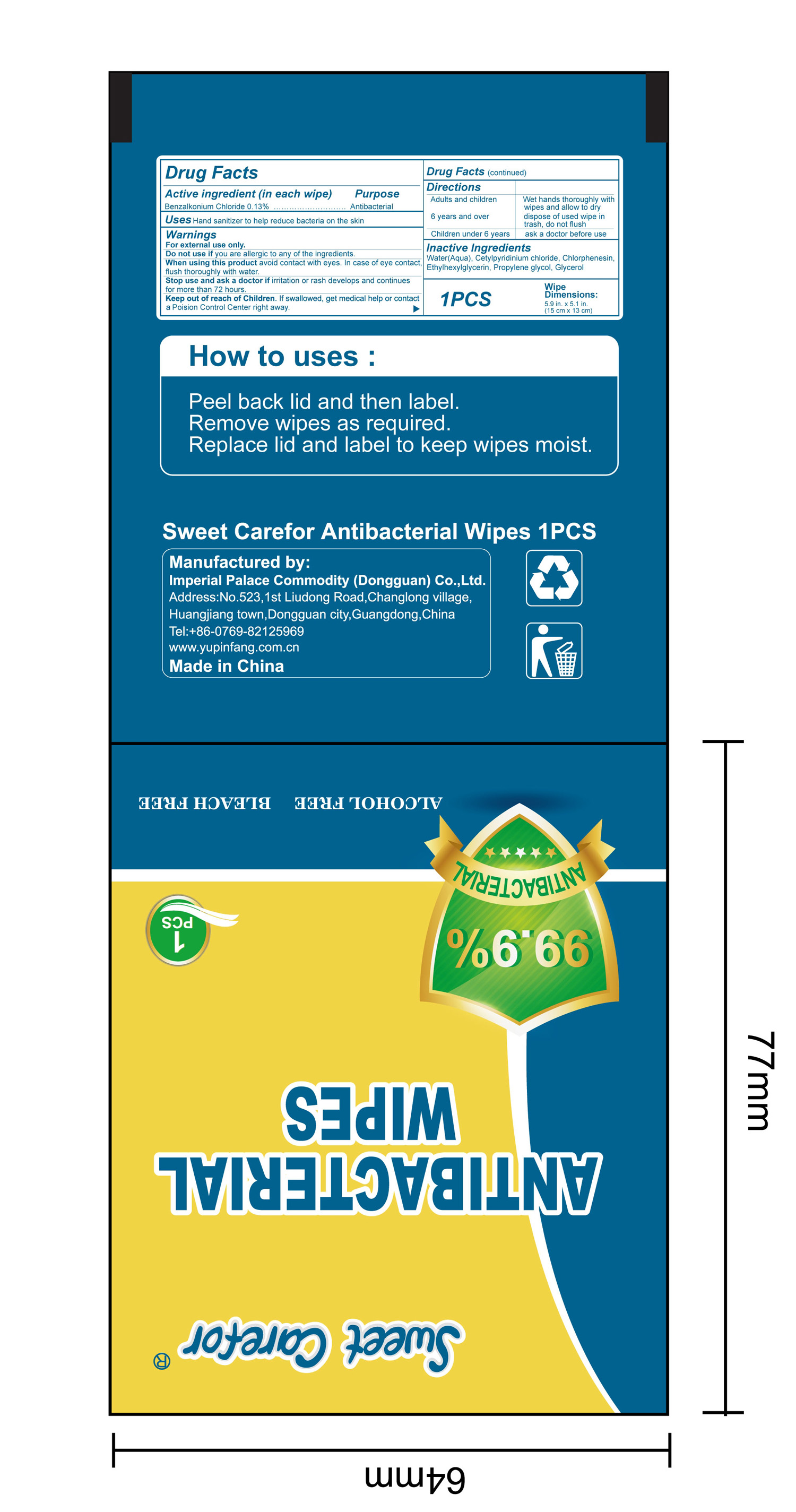 NDC:52489-002-07
NDC:52489-002-07
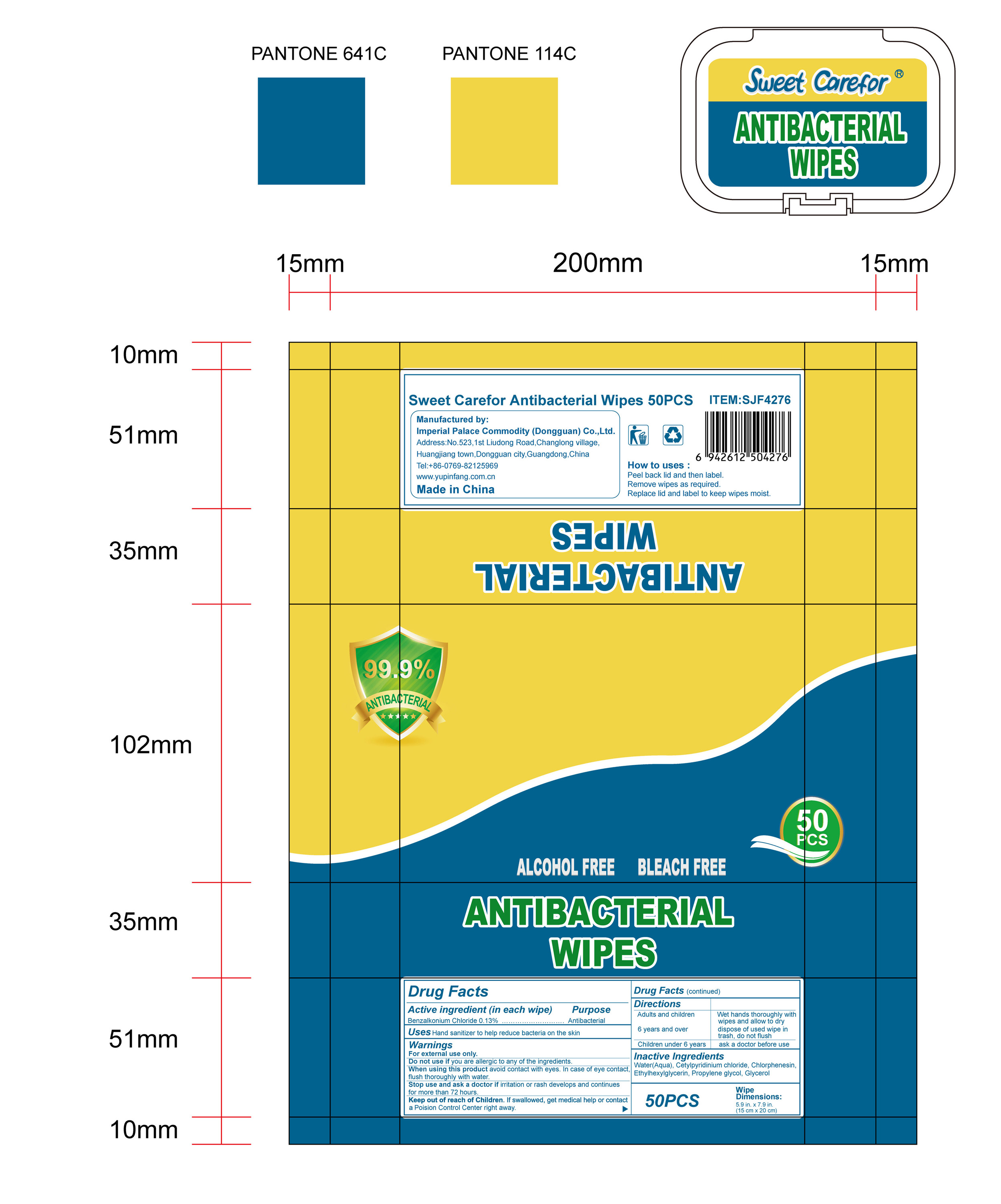 NDC:52489-002-08
NDC:52489-002-08