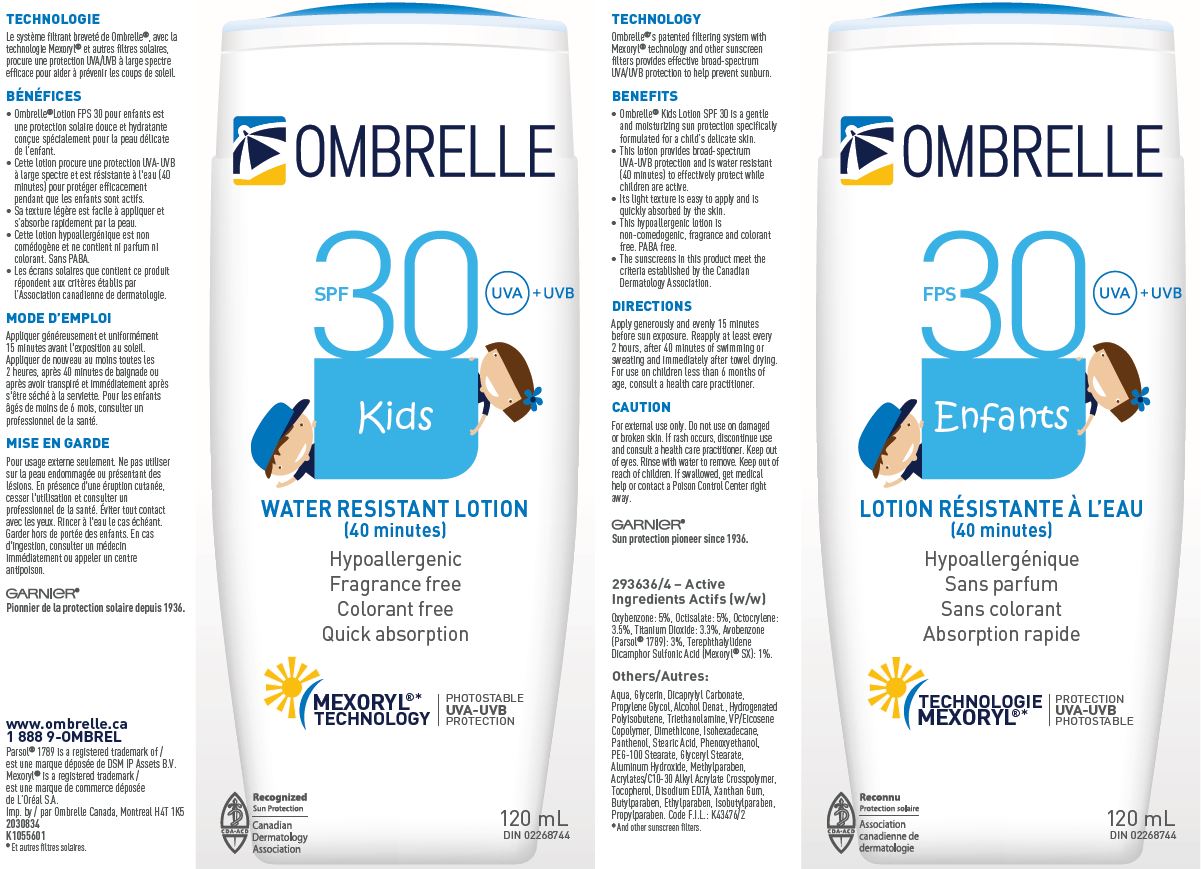
GARNIER OMBRELLE SPF 30 KIDS WATER RESISTANT- oxybenzone, octisalate, octocrylene, titanium dioxide, avobenzone and terephthalylidene dicamphor sulfonic acid lotion
L'Oreal USA Products Inc
----------
Drug Facts
Active ingredients
Oxybenzone 5%
Octisalate 5%
Octocrylene 3.5%
Titanium Dioxide 3.3%
Avobenzone 3%
Terephthalylidene Dicamphor Sulfonic Acid 1%
Caution
For external use only. Do not use on damaged or broken skin. If rash occurs, discontinue use and consult a health care practitioner. Keep out of eyes. Rinse with water to remove. Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Apply generously and evenly 15 minutes before sun exposure. Reapply at least every 2 hours, after 40 minutes of swimming or sweating and immediately after towel drying. For use of children less than 6 months of age, consult a health care practitioner.
Others
Aqua, Glycerin, Dicaprylyl Carbonate, Propylene Glycol, Alcohol Denat., Hydrogenated Polyisobutene, Triethanolanime, VP/Eicosene Copolymer, Dimethicone, Isohexadecane, Panthenol, Stearic Acid, Phenoxyethanol, PEG-100 Stearate, Glyceryl Stearate, Aluminum Hydroxide, Methylparaben, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Tocopherol, Disodium EDTA, Xanthan Gum, Butylparaben, Ethylparaben, Isobutylparaben, Propylparaben
| GARNIER OMBRELLE SPF 30 KIDS WATER RESISTANT
oxybenzone, octisalate, octocrylene, titanium dioxide, avobenzone and terephthalylidene dicamphor sulfonic acid lotion |
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - L'Oreal USA Products Inc (002136794) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| L'Oreal USA, Inc. | 624244349 | manufacture(49967-462) , pack(49967-462) | |