COLD AND FLU NIGHT- acetaminophen, dextromethorphan hbr, doxylamine succinate, phenylephrine hcl tablet, film coated
Chalkboard Health Inc
----------
Fluent 44-677 Delisted
Active ingredients (in each caplet)
Acetaminophen 325 mg
Dextromethorphan HBr 10 mg
Doxylamine succinate 6.25 mg
Phenylephrine HCl 5 mg
Uses
- temporarily relieves common cold and flu symptoms:
- minor aches and pains
- sinus congestion and pressure
- sore throat
- fever
- headache
- nasal congestion
- cough due to minor throat and bronchial irritation
- runny nose and sneezing
- reduces swelling of nasal passages
- promotes nasal and/or sinus drainage
- temporarily restores freer breathing through the nose
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
- more than 4,000 mg of acetaminophen in 24 hours
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- blisters
- rash
- skin reddening
If a skin reaction occurs, stop use and seek medical help right away.
Sore throat warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- if you have ever had an allergic reaction to this product or any of its ingredients
Ask a doctor before use if you have
- liver disease
- diabetes
- thyroid disease
- heart disease
- glaucoma
- high blood pressure
- difficulty in urination due to enlargement of the prostate gland
- a breathing problem or chronic cough that lasts or as occurs with smoking, asthma, chronic bronchitis, or emphysema
- cough that occurs with too much phlegm (mucus)
Ask a doctor or pharmacist before use if you are
- taking sedatives or tranquilizers
- taking the blood thinning drug warfarin
When using this product
- do not exceed recommended dosage
- excitability may occur, especially in children
- marked drowsiness may occur
- avoid alcoholic beverages
- be careful when driving a motor vehicle or operating machinery
- alcohol, sedatives, and tranquilizers may increase drowsiness
Stop use and ask a doctor if
- nervousness, dizziness, or sleeplessness occur
- pain, nasal congestion, or cough gets worse or lasts more than 7 days
- fever gets worse or lasts more than 3 days
- redness or swelling is present
- new symptoms occur
- cough comes back or occurs with rash or headache that lasts. These could be signs of a serious condition.
Directions
- do not take more than directed
- do not take more than 8 caplets in 24 hours
- adults and children 12 years and over: take 2 caplets with water every 4 hours
- children under 12 years: ask a doctor
Other information
- TAMPER EVIDENT: DO NOT USE IF OUTER PACKAGE IS OPENED OR BLISTER IS TORN OR BROKEN
- store at 25°C (77°F); excursions permitted between 15°-30°C (59°-86°F)
- see end flap for expiration date and lot number
Inactive ingredients
black iron oxide, corn starch, crospovidone, D&C yellow #10 aluminum lake, FD&C blue #1 aluminum lake, FD&C yellow #6 aluminum lake, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polysorbate 80, polyvinyl alcohol, povidone, silicon dioxide, sodium starch glycolate, stearic acid, talc, titanium dioxide
Principal display panel
ºFluent™
NDC 79395-677-08
Cold+Flu
Severe NIGHT
Acetaminophen
Dextromethorphan HBr
Doxylamine succinate
Phenylephrine HCl
Pain Reliever/Fever Reducer
Cough Suppressant
Antihistamine
Nasal Decongestant
24 Caplets/Actual Size
TAMPER EVIDENT: DO NOT USE IF
PACKAGE IS OPENED OR IF
BLISTER UNIT IS TORN, BROKEN
OR SHOWS ANY SIGNS OF
TAMPERING
PARENTS:
Learn about teen medicine abuse
www.StopMedicineAbuse.org
Distributed By:
Chalkboard Health, Inc.
Princeton, NJ 08540
getfluenthealth.com
50844 ORG051967708
44-677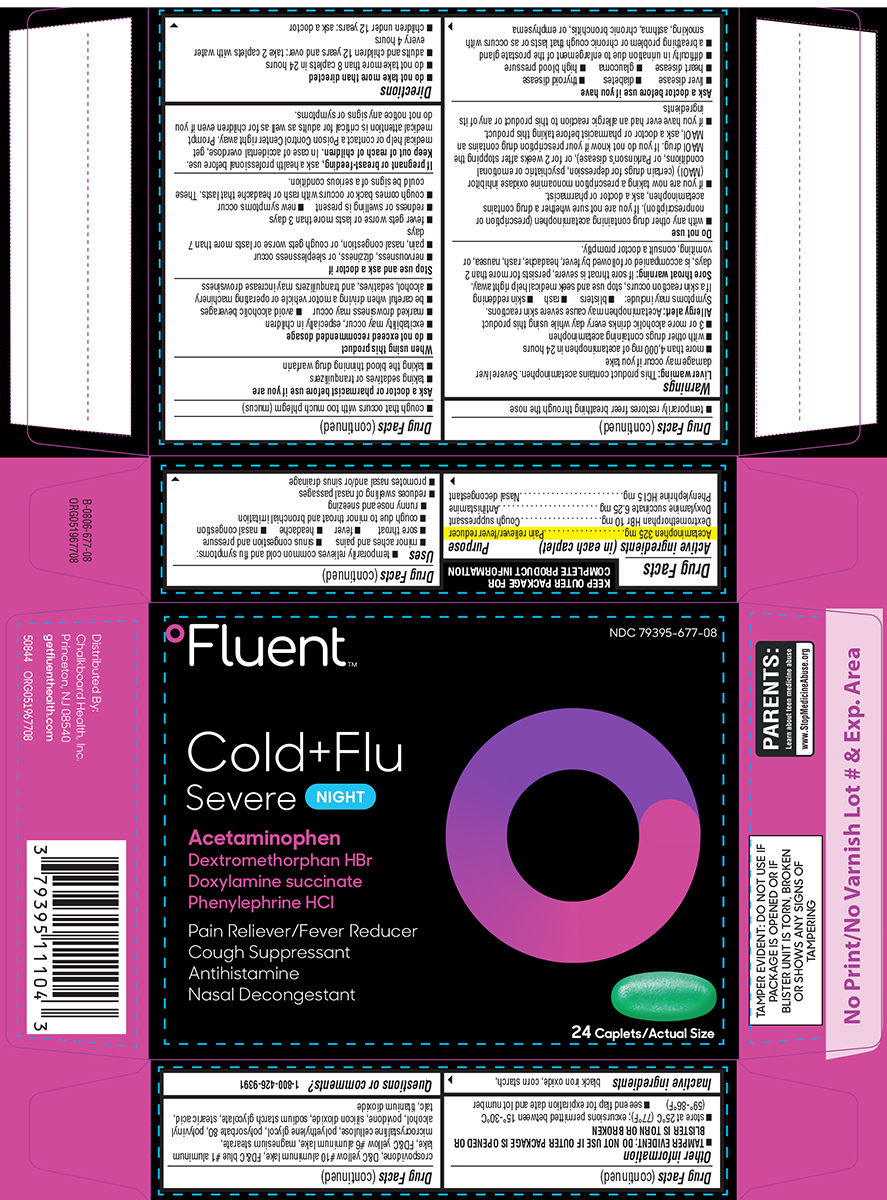
| COLD AND FLU
NIGHT
acetaminophen, dextromethorphan hbr, doxylamine succinate, phenylephrine hcl tablet, film coated |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| Labeler - Chalkboard Health Inc (117569507) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867837 | manufacture(79395-677) , pack(79395-677) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867894 | manufacture(79395-677) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 868734088 | manufacture(79395-677) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 117025878 | manufacture(79395-677) | |