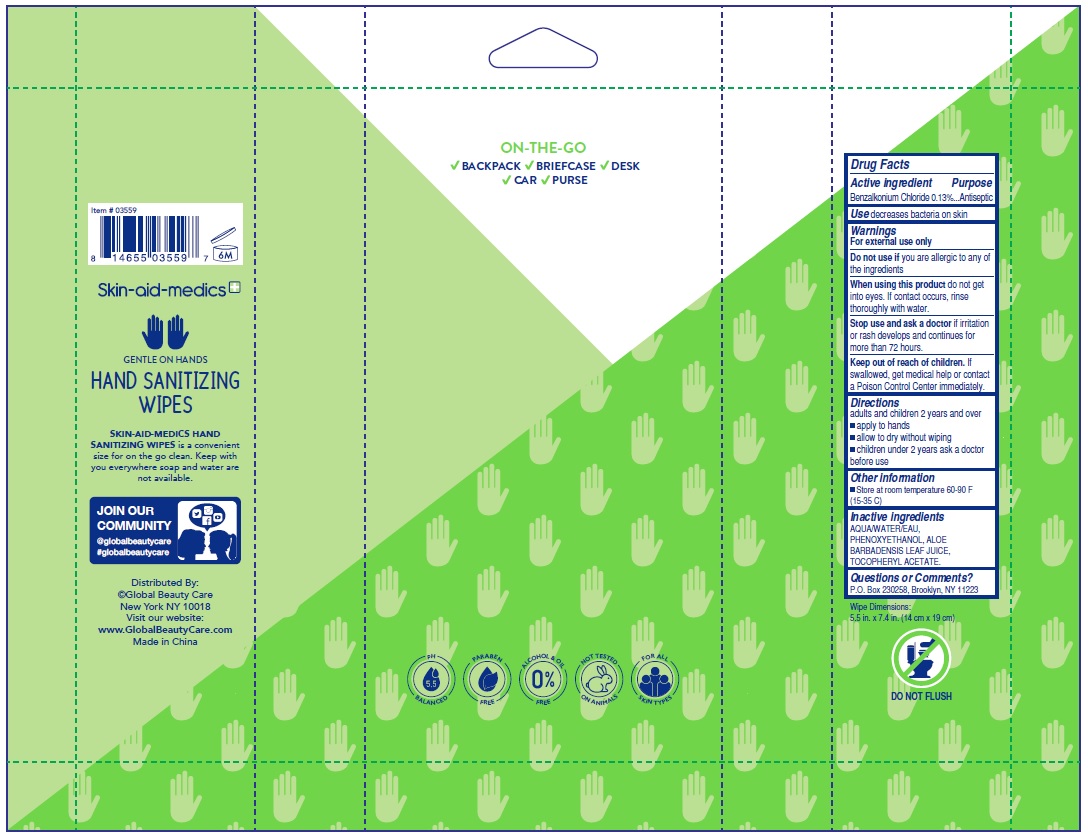
SKIN-AID- MEDICS ANTIBACTERIAL WIPES FRAGRANCE FREE- benzalkonium chloride 0.13% swab
Kangna (Zhejiang) Medical Supplies Co., Ltd
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Skin-aid-Medics Antibacterial Wipes Fragrance-Free
Stop use and ask a doctor.
If irritation or rash develops and continues for more than 72 hours.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center immediately.
| SKIN-AID- MEDICS ANTIBACTERIAL WIPES FRAGRANCE FREE
benzalkonium chloride 0.13% swab |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Kangna (Zhejiang) Medical Supplies Co., Ltd (554530173) |
| Registrant - Global Beauty Care, Inc (068600947) |
Revised: 7/2021
Document Id: c849b14f-325f-2573-e053-2995a90a00ea
Set id: a6192d6f-a468-37c2-e053-2a95a90ab8a9
Version: 2
Effective Time: 20210729
Kangna (Zhejiang) Medical Supplies Co., Ltd