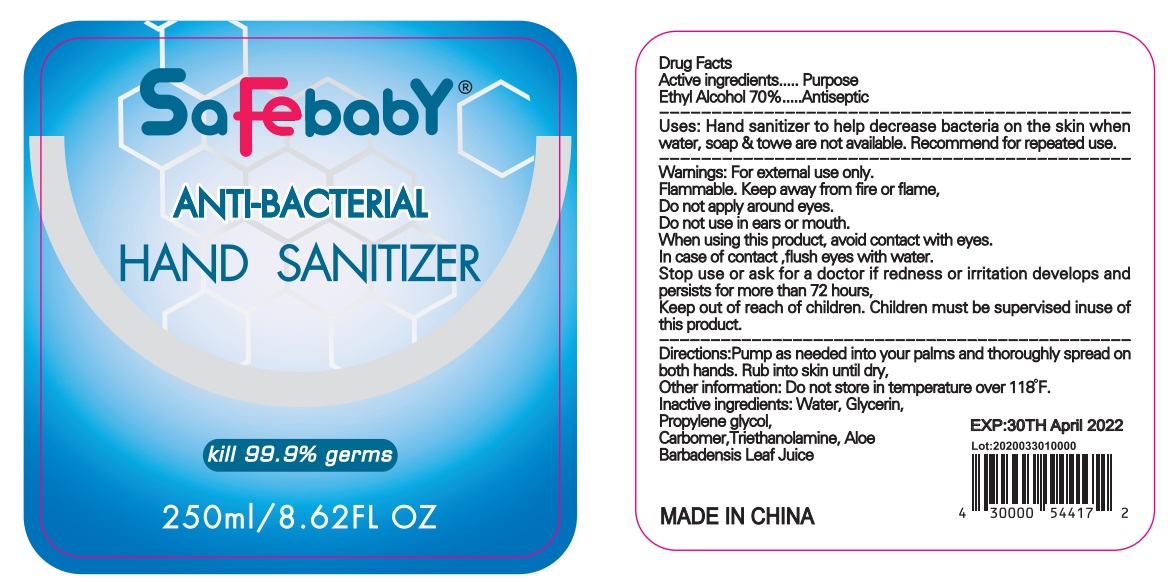
ANTI-BACTERIAL HAND SANITIZER- anti-bacterial hand sanitizer liquid
Dongguan Benoy Industrial CO.,LTD
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
ANTI-BACTERIAL HAND SANITIZER
Use
Hand sanitizer to help decrease bacteria on the skin when water, soap &u to we are not available Recommend for repeated use
Directions
Pump as needed into our palms and thoroughly spread on both hands. Rub into skin until dry
| ANTI-BACTERIAL HAND SANITIZER
anti-bacterial hand sanitizer liquid |
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| Labeler - Dongguan Benoy Industrial CO.,LTD (403972510) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Dongguan Benoy Industrial CO.,LTD | 403972510 | manufacture(75387-501) | |
Revised: 1/2021
Document Id: b963d83b-584e-c447-e053-2995a90ad973
Set id: a60c5ff5-b8f4-1f0b-e053-2995a90a67a3
Version: 2
Effective Time: 20210121
Dongguan Benoy Industrial CO.,LTD