Label: GENTEAL TEARS SEVERE- hypromellose gel
- NDC Code(s): 0065-8064-01
- Packager: Alcon Laboratories, Inc.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated December 14, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- Uses
- Warnings
- Do not use
- When using this product
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other information
- Inactive ingredients
- Questions?
-
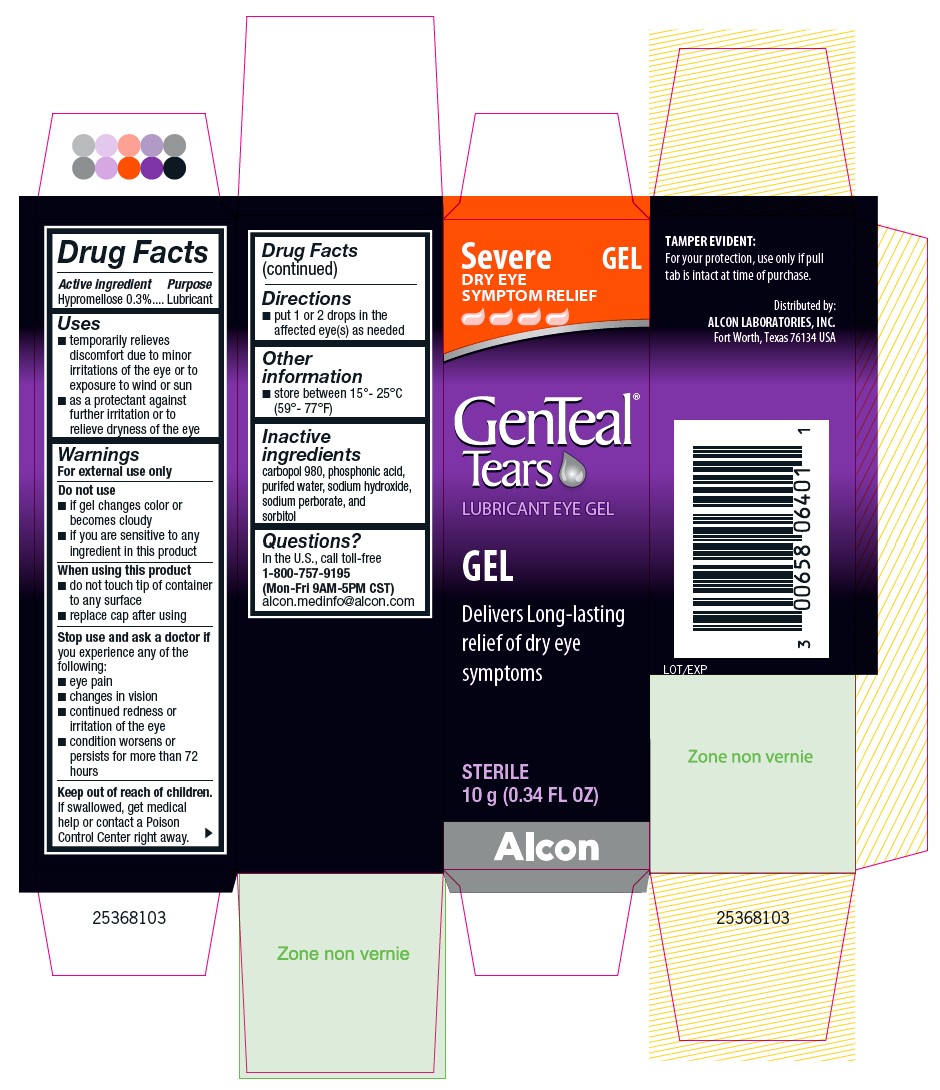
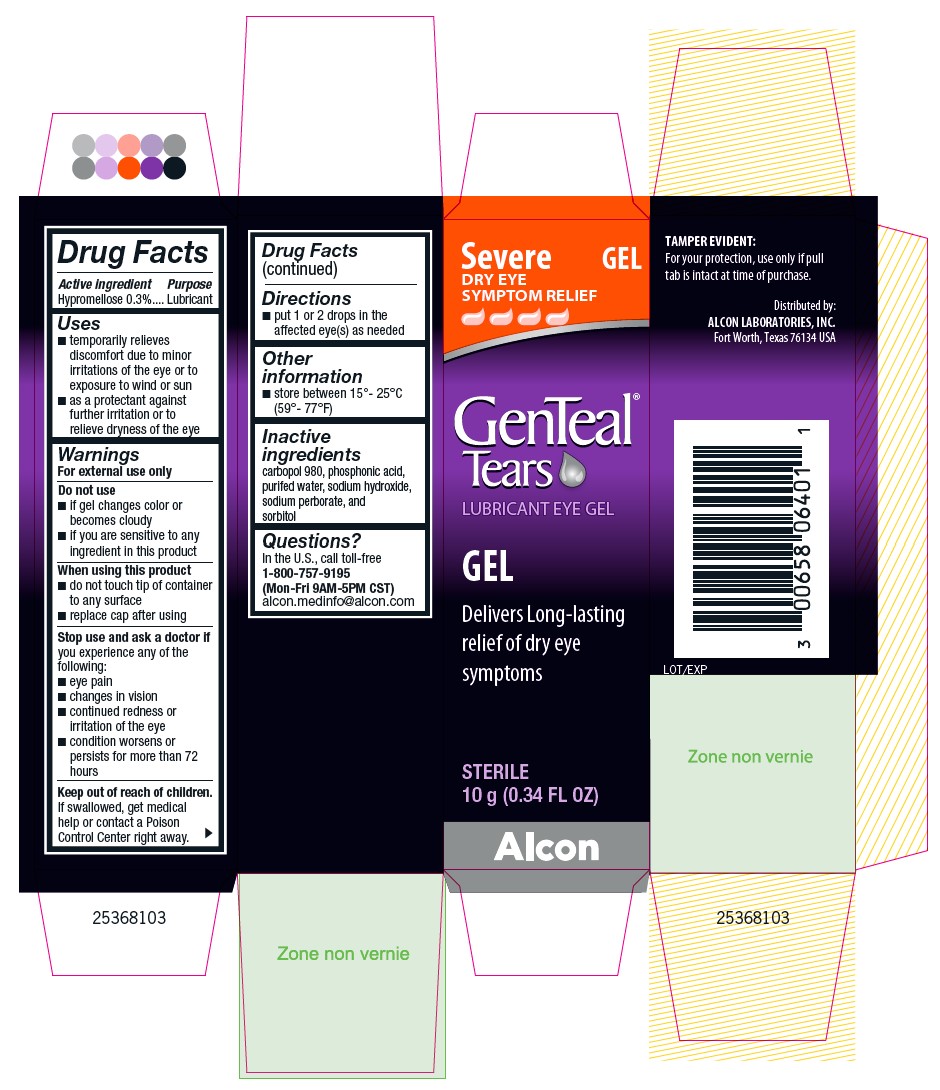
PRINCIPAL DISPLAY PANEL
Severe DRY EYE SYMPTOM RELIEF
GEL
GenTeal® Tears
LUBRICANT EYE GEL
GEL
Delivers Long-lasting relief of dry eye symptoms
STERILE
10 g (0.34 FL OZ)
TAMPER EVIDENT:
For your protection, use only if pull tab is intact at time of purchase.
Distributed by:
ALCON LABORATORIES, INC.
Fort Worth, Texas 76134 USA
Lot/Exp
Alcon
25368103
Severe DRY EYE SYMPTOM RELIEF
GenTeal® Tears
LUBRICANT EYE GEL
GEL
Delivers long-lasting relief of dry eye symptoms
STERILE 10g (0.34 FL OZ)
Active Ingredient: Hypromellose (0.3%) Uses: See carton for Uses.
Warnings: See carton for Warnings. When using this product do not touch tip of container to any surface. Replace cap after using. Directions: Put 1 or 2 drops in the affected eye(s) as needed. Other Information: Store between 15°-25°C (59°-77°F).
TAMPER EVIDENT: For your protection, use only if pull tab is intact at the time of purchase.
Distributed by:
ALCON LABORATORIES, INC.
6201 South Freeway, Fort Worth, Texas 76134 USA
ALCON
16027802
-
INGREDIENTS AND APPEARANCE
GENTEAL TEARS SEVERE
hypromellose gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0065-8064 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Hypromellose 2910 (4000 Mpa.S) (UNII: RN3152OP35) (Hypromellose 2910 (4000 Mpa.S) - UNII:RN3152OP35) Hypromellose 2910 (4000 Mpa.S) .003 g in 1 g Inactive Ingredients Ingredient Name Strength Sodium Perborate (UNII: Y52BK1W96C) Phosphonic Acid (UNII: 35V6A8JW8E) Water (UNII: 059QF0KO0R) Sodium Hydroxide (UNII: 55X04QC32I) Sorbitol (UNII: 506T60A25R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0065-8064-01 1 in 1 CARTON 12/31/2019 1 10 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M018 12/31/2019 Labeler - Alcon Laboratories, Inc. (008018525) Establishment Name Address ID/FEI Business Operations Excelvision 274234566 manufacture(0065-8064) , label(0065-8064) , pack(0065-8064)