TOO IM CELL ALL IN ONE- glycerin, niacinamide, adenosine lotion
Pharmacal-International. Co., Ltd
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Warning
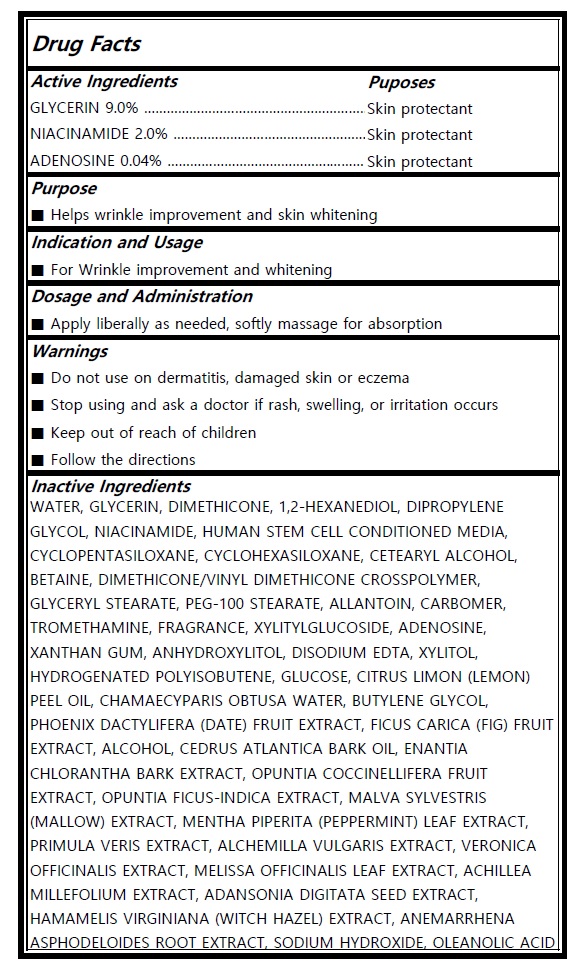
■ Do not use on dermatitis, damaged skin or eczema
■ Stop using and ask a doctor if rash, swelling, or irritation occurs
■ Keep out of reach of children
■ Follow the directions
Purpose
■ Helps wrinkle improvement and skin whitening
Dosage and Administration
■ Apply liberally as needed, softly massage for absorption
Indication and Usage
■ For Wrinkle improvement and whitening
Keep out of reach of children
Active Ingredients
GLYCERIN 9.0%
NIACINAMIDE 2.0%
ADENOSINE 0.04%
Inactive Ingredients
WATER, GLYCERIN, DIMETHICONE, 1,2-HEXANEDIOL, DIPROPYLENE
GLYCOL, NIACINAMIDE, HUMAN STEM CELL CONDITIONED MEDIA,
CYCLOPENTASILOXANE, CYCLOHEXASILOXANE, CETEARYL ALCOHOL,
BETAINE, DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER,
GLYCERYL STEARATE, PEG-100 STEARATE, ALLANTOIN, CARBOMER,
TROMETHAMINE, FRAGRANCE, XYLITYLGLUCOSIDE, ADENOSINE,
XANTHAN GUM, ANHYDROXYLITOL, DISODIUM EDTA, XYLITOL,
HYDROGENATED POLYISOBUTENE, GLUCOSE, CITRUS LIMON (LEMON)
PEEL OIL, CHAMAECYPARIS OBTUSA WATER, BUTYLENE GLYCOL,
PHOENIX DACTYLIFERA (DATE) FRUIT EXTRACT, FICUS CARICA (FIG) FRUIT
EXTRACT, ALCOHOL, CEDRUS ATLANTICA BARK OIL, ENANTIA
CHLORANTHA BARK EXTRACT, OPUNTIA COCCINELLIFERA FRUIT
EXTRACT, OPUNTIA FICUS-INDICA EXTRACT, MALVA SYLVESTRIS
(MALLOW) EXTRACT, MENTHA PIPERITA (PEPPERMINT) LEAF EXTRACT,
PRIMULA VERIS EXTRACT, ALCHEMILLA VULGARIS EXTRACT, VERONICA
OFFICINALIS EXTRACT, MELISSA OFFICINALIS LEAF EXTRACT, ACHILLEA
MILLEFOLIUM EXTRACT, ADANSONIA DIGITATA SEED EXTRACT,
HAMAMELIS VIRGINIANA (WITCH HAZEL) EXTRACT, ANEMARRHENA
ASPHODELOIDES ROOT EXTRACT, SODIUM HYDROXIDE, OLEANOLIC ACID
Image

Pharmacal-International. Co., Ltd

