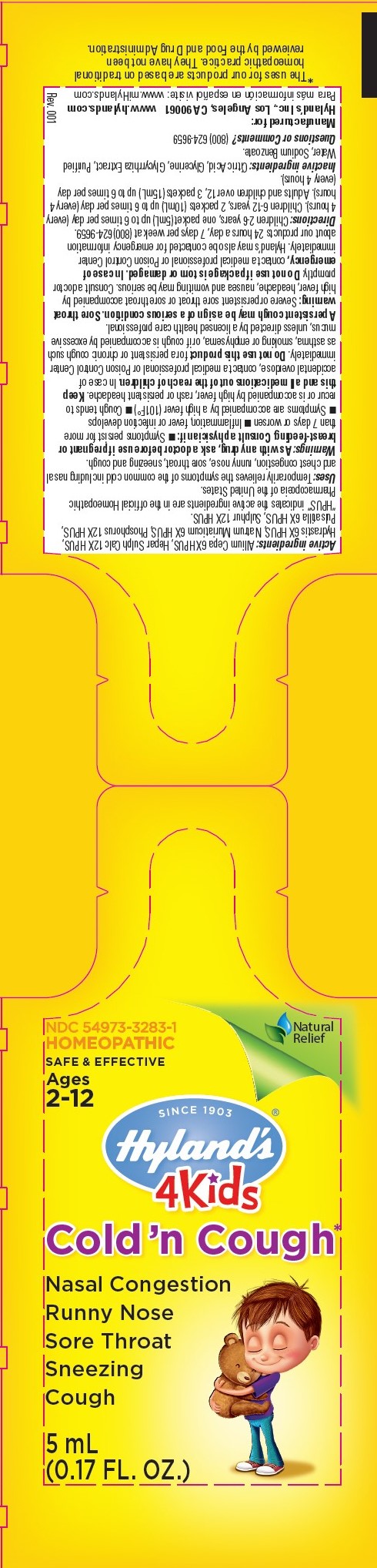
4 KIDS COLD N COUGH- onion, calcium sulfide, goldenseal, sodium chloride, phosphorus, anemone pulsatilla, and sulfur liquid
Hyland's
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
4 Kids Cold 'n Cough
Active ingredients
Allium Cepa 6X HPUS, Hepar Sulph Calc 12X HPUS, Hydrastis 6X HPUS, Natrum Muriaticum 6X HPUS, Phosphorus 12X HPUS, Pulsatilla 6X HPUS, Sulphur 12X HPUS.
"HPUS" indicates the active ingredients are in the official Homeopathic Pharmacopœia of the United States.
Warnings
As with any drug, ask a doctor before use if pregnant or breast-feeding. Consult a physician if:
- Symptoms persist for more than 7 days or worsen
- Inflammation, fever or infection develops
- Symptoms are accompanied by a high fever (101F°)
- Cough tends to recur or is accompanied by high fever, rash or persistent headache.
Keep this and all medications out of the reach of children. In case of accidental overdose, contact a medical professional or Poison Control Center immediately. Do not use this product for a persistent or chronic cough such as asthma, smoking or emphysema, or if cough is accompanied by excessive mucus, unless directed by a licensed health care professional.
A persistent cough may be a sign of a serious condition. Sore throat warning: Severe or persistent sore throat or sore throat accompanied by high fever, headache, nausea and vomiting may be serious. Consult a doctor promptly. Do not use if package is torn or damaged. In case of emergency, contact a medical professional or Poison Control Center immediately. Hyland's may also be contacted for emergency information about our products 24 hours a day, 7 days per week at (800) 624-9659.
| 4 KIDS COLD N COUGH
onion, calcium sulfide, goldenseal, sodium chloride, phosphorus, anemone pulsatilla, and sulfur liquid |
|||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
| Labeler - Hyland's (028570695) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Standard Homeopathic Company | 008316655 | manufacture(54973-3283) , pack(54973-3283) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Applied Laboratories, Inc. | 876754375 | manufacture(54973-3283) , pack(54973-3283) | |