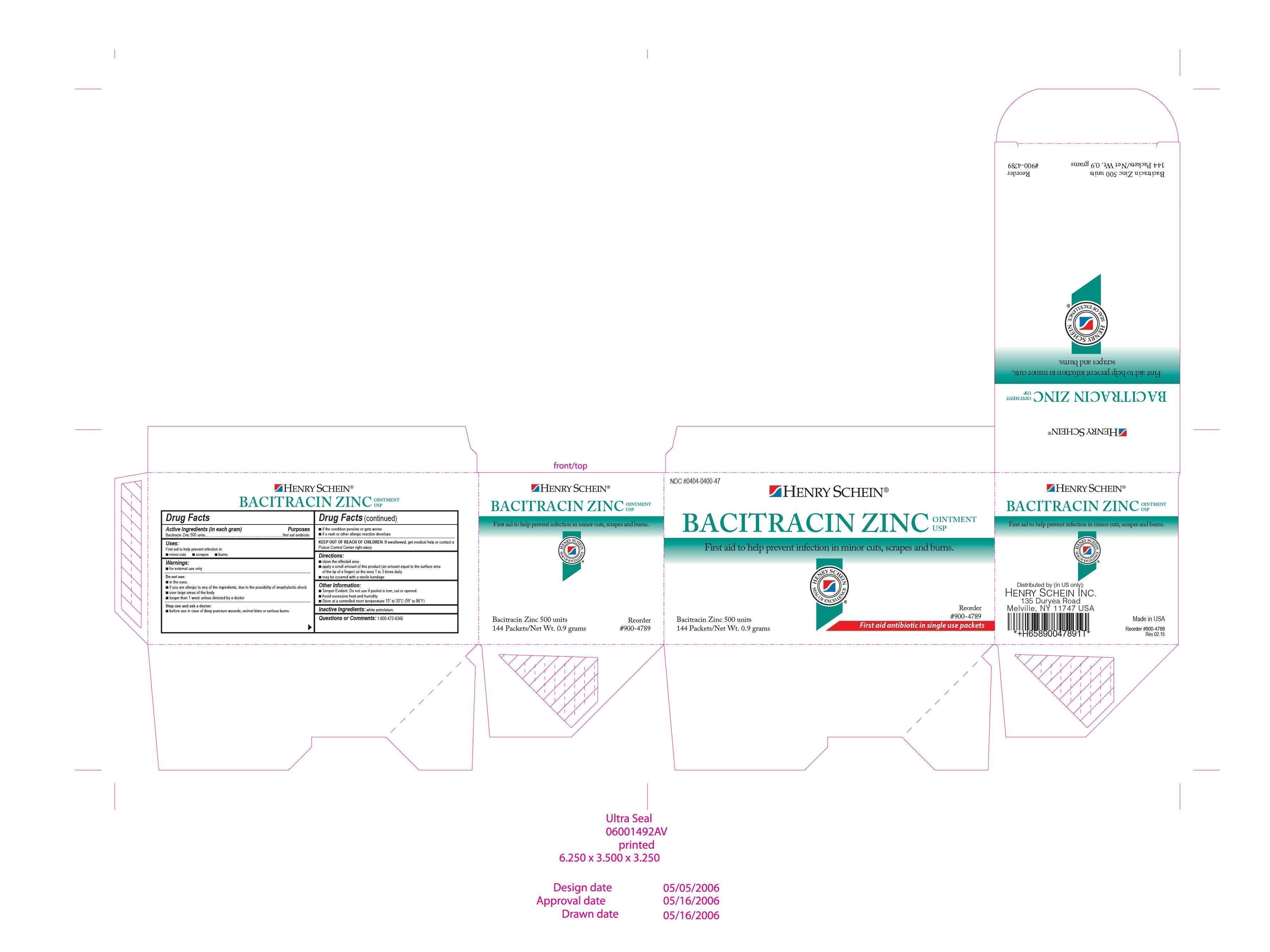
SCHEIN BACITRACIN- bacitracin zinc ointment
Henry Schein, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
DRUG FACTS
DO NOT USE: In the eyes. Over large areas of the body. If you are allergic to any of the ingredients due to the possibility of anaphylactic shock. Longer than 1 week unless directed by a doctor.
Stop use and ask a doctor before use: in the case of dep puncture wounds, animal bites, or serious burns. If the condition persists or gets worse. If a rash or other allergic reaction develops.
If swallowed get medical help or contact a Poison Control Center immediately.
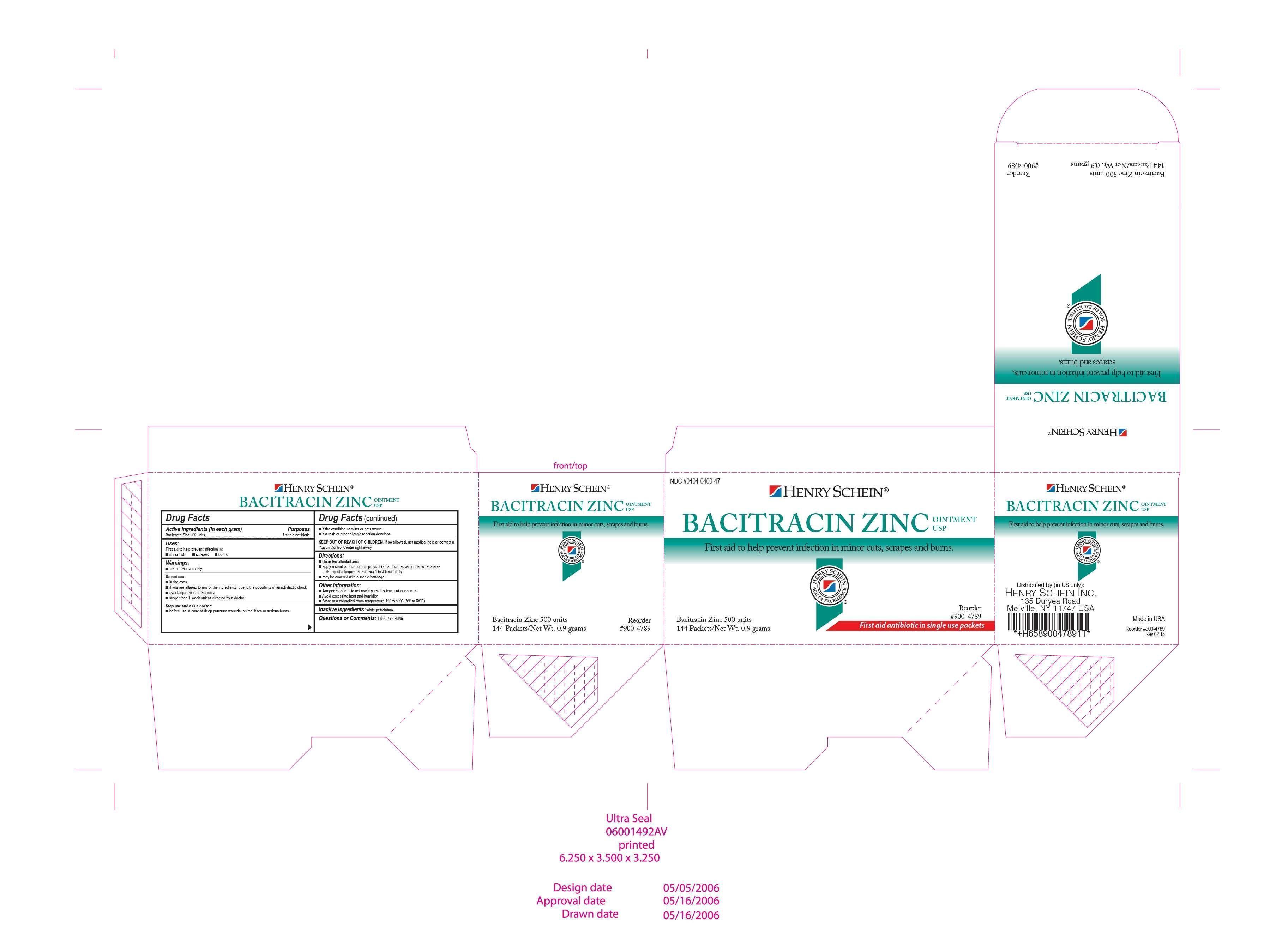
| SCHEIN BACITRACIN
bacitracin zinc ointment |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Henry Schein, Inc. (012430880) |
| Registrant - ULTRAtab Laboratories, Inc. (151051757) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Ultra Seal Corporation | 085752004 | pack(0404-0400) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| ULTRAtab Laboratories, Inc. | 151051757 | manufacture(0404-0400) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Ultra Seal Corporation | 944090448 | pack(0404-0400) | |
Revised: 1/2023
Document Id: f1634ac6-dd80-1580-e053-2995a90a842a
Set id: a523e36e-121e-43e4-ae5f-377ba76a2d86
Version: 9
Effective Time: 20230103
Henry Schein, Inc.