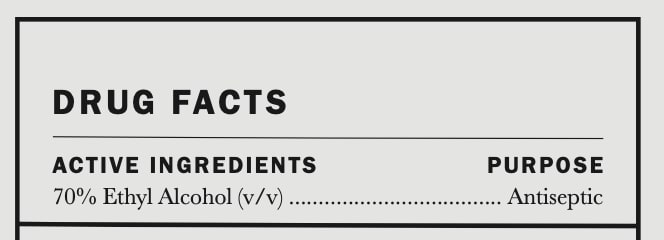
AMASS BOTANICS HAND SANITIZER- amass botanics hand saniziter liquid
AMASS Brands, Inc
----------
Warnings: Do Not Use
Do not use in or near the eyes. In case of contact, rinse thoroughly with water.
Stop use and ask a doctor if irritation or rash occurs.
Keep out of reach of children. If swallowed, get medical attention or contact a poison center immediately.

Warnings: Keep out of reach of children section
Keep out of reach of children. If swallowed, get medical attention or contact a poison center immediately.

Directions when using
DIRECTIONS
Apply liberally to dry hands, rub briskly until evaporated.
Children should be supervised when using this product.

Warnings
Flammable. Keep away from heat or flame.
For external use only.
Do not use in or near the eyes. In case of contact, rinse thoroughly with water.
Stop use and ask a doctor if irritation or rash occurs.
Keep out of reach of children. If swallowed, get medical attention or contact a poison center immediately.

Directions
DIRECTIONS
Apply liberally to dry hands, rub briskly until evaporated.
Children should be supervised when using this product.

INACTIVE INGREDIENTS
Aloe Barbadensis Leaf Juice, Glycerin, Cinnamomum Verum (Cinnamon), Pimenta Diocia (Allspice), Syzygium Aromaticum (Clove), Eucalyptus Obliqua (Eucalyptus).

DIRECTIONS
Apply liberally to dry hands, rub briskly until evaporated.
Children should be supervised when using this product.

WARNINGS
Flammable. Keep away from heat or flame.
For external use only.
Do not use in or near the eyes. In case of contact, rinse thoroughly with water.
Stop and use and ask a doctor if irritation or rash occurs.
Keep out of reach of children. If swallowed, get medical attention or contact a poison center immediately.

| AMASS BOTANICS HAND SANITIZER
amass botanics hand saniziter liquid |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - AMASS Brands, Inc (121284034) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| AMASS Brands, Inc | 121284034 | manufacture(77419-0001) | |