PNV TABS 29-1- .beta.-carotene, ascorbic acid, cholecalciferol, .alpha.-tocopherol acetate, dl-, thiamine mononitrate, riboflavin, niacinamide, pyridoxine hydrochloride, folic acid, cyanocobalamin, biotin, calcium pantothenate, calcium carbonate, iron, potassium iodide, magnesium oxide, zinc oxide, and cupric oxide tablet
Virtus Pharmaceuticals
----------
PNV Tabs 29-1
DESCRIPTION
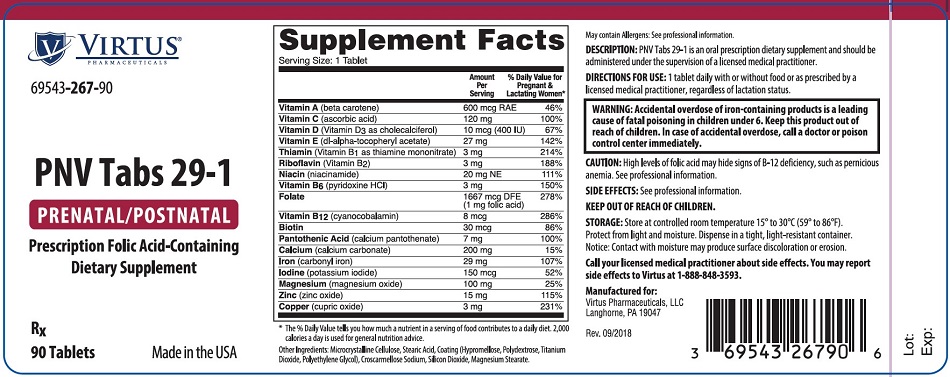
PNV Tabs 29-1 is a prescription oral dietary supplement and should be administered under the supervision of a licensed medical practitioner.
| Supplement Facts Table
Serving Size: 1 Tablet |
|||
|---|---|---|---|
| Amount Per Serving | % Daily Value* for Pregnant &
Lactating Women |
||
|
|||
|
Vitamin A (beta carotene) |
600 mcg RAE |
46 % | |
|
Vitamin C (ascorbic acid) |
120 mg |
100 % | |
|
Vitamin D (Vitamin D3 as cholecalciferol) |
10 mcg (400 IU) |
67 % | |
|
Vitamin E (dl-alpha-tocopheryl acetate) |
27 mg |
142 % | |
|
Thiamin (Vitamin B1 as thiamine mononitrate) |
3 mg |
214 % | |
|
Riboflavin (Vitamin B2) |
3 mg |
188 % | |
|
Niacin (niacinamide) |
20 mg NE |
111 % | |
|
Vitamin B6 (pyridoxine HCl) |
3 mg |
150 % | |
|
Folate |
1667 mcg DFE |
278 % | |
|
Vitamin B12 (cyanocobalamin) |
8 mcg |
286 % | |
|
Biotin |
30 mcg |
86 % | |
|
Pantothenic Acid (calcium pantothenate) |
7 mg |
100 % | |
|
Calcium (calcium carbonate) |
200 mg |
15 % | |
|
Iron (carbonyl iron) |
29 mg |
107 % | |
|
Iodine (potassium iodide) |
150 mcg |
52 % | |
|
Magnesium (magnesium oxide) |
100 mg |
25 % | |
|
Zinc (zinc oxide) |
15 mg |
115 % | |
|
Copper (cupric oxide) |
3 mg |
231 % | |
Other Ingredients: Microcrystalline Cellulose, Stearic Acid, Coating (Hypromellose, Polydextrose, Titanium Dioxide, Polyethylene Glycol), Croscarmellose Sodium, Silicon Dioxide, Magnesium Stearate.
ALLERGY STATEMENT
This product has been manufactured in a facility that also manufactures products containing tree nuts, peanuts, fish, egg, wheat, milk, soy and shellfish. Individuals with allergic tendencies to these substances should use discretion.
PHARMACOLOGY
Adequate Folate in healthful diets may reduce a woman's risk of having a child with a brain or spinal cord defect.1
SIDE EFFECTS2
Side effects are uncommon with folic acid, thiamine, riboflavin, pyridoxine, iron, and copper.
Gastrointestinal side effects including anorexia, nausea, abdominal distention, flatulence, and bitter taste are rarely seen with folic acid and pyridoxine. Gastrointestinal side effects due to iron include nausea, constipation, anorexia, heartburn, vomiting, and diarrhea.
Hypersensitivity reactions due to folic, thiamine, or pyridoxine have been seen rarely.
PATIENT INFORMATION
This product is a dietary management prescription folate supplement to be used only under licensed medical supervision. Consult a medical practitioner before use with medications.
KEEP THIS PRODUCT OUT OF THE REACH OF CHILDREN.
INTERACTIONS2
Folic acid has major interactions with fluorouracil and its prodrugs, for example capecitabine. Use of this product with patients who are taking fluorouracil drugs should be undertaken only with extreme caution under the direction of a licensed medical practitioner.
Folic acid also interacts moderately with antiepileptic drugs, monoamines (e.g. levodopa, serotonin) and drugs that interfere with homocysteine metabolism.
Drugs known to interfere with folic acid absorption from the intestine include cholestyramine, colestipol, and cyclosporine.
Drugs which may interact moderately with vitamin B12 include arsenic and chloramphenicol.
Carbonyl iron has major interactions with abacavir/dolutegravir and lamivudine, dimercaprol and dolutegravir.
Vitamin C (ascorbic acid) interacts moderately with amphetamines and the antineoplastic drug bortezomib.
CONTRAINDICATIONS
This product is contraindicated in patients with a known hypersensitivity to any of the components contained in this product. This product is contraindicated for individuals with conditions for which any of the ingredients are contraindicated.
|
Warning: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. Keep this product out of reach of children. In case of accidental overdose, call a doctor or poison control center immediately3. |
DIRECTIONS FOR USE
One tablet daily with or without food or as prescribed by a licensed medical practitioner, regardless of lactation status.
REFERENCES
- 1.
- 21 CFR §101.79(d)(1)(ii)
- 2.
- All side effects and drug information was obtained from www.drugs.com
- 3.
- 21 CFR 101.17(e)(1)
PREGNANCY and NURSING MOTHERS
Use of PNV Tabs 29-1 with products containing folic acid is not recommended, as this will result in folic acid intake that exceeds the daily upper limit.
STORAGE
Store at controlled room temperature 15° to 30°C (59° to 86°F). Protect from light and moisture. Dispense in a tight, light-resistant container. Notice: Contact with moisture may produce surface discoloration or erosion.
HOW SUPPLIED
PNV Tabs 29-1 are supplied as oval, white tablets debossed on one side with "V267" dispensed in child-resistant bottles of 90 tablets.
Call your medical practitioner about side effects. You may report side effects by calling Virtus at 1-888-848-3593.
Rx
Manufactured for:
Virtus Pharmaceuticals, LLC.
Langhorne, PA 19047
MADE IN USA
Rev. 09/2018
| PNV TABS 29-1
.beta.-carotene, ascorbic acid, cholecalciferol, .alpha.-tocopherol acetate, dl-, thiamine mononitrate, riboflavin, niacinamide, pyridoxine hydrochloride, folic acid, cyanocobalamin, biotin, calcium pantothenate, calcium carbonate, iron, potassium iodide, magnesium oxide, zinc oxide, and cupric oxide tablet |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Supplement Facts | ||
| Serving Size : | Serving per Container : | |
| Amount Per Serving | % Daily Value | |
|---|---|---|
| color | ||
| size (solid drugs) | 19 mm | |
| shape | ||
| scoring | 1 | |
| imprint | ||
| Labeler - Virtus Pharmaceuticals (079659493) |