Label: AEROWASH EYEWASH- eyewash solution liquid
- NDC Code(s): 55305-115-10, 55305-115-11, 55305-115-12, 55305-115-13
- Packager: Aero Healthcare US LLC
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated March 6, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
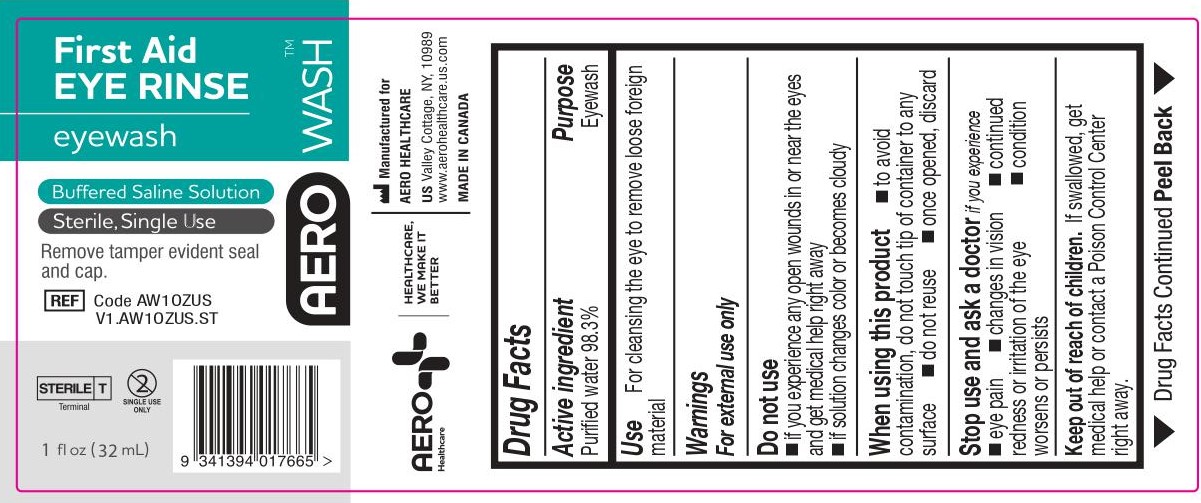
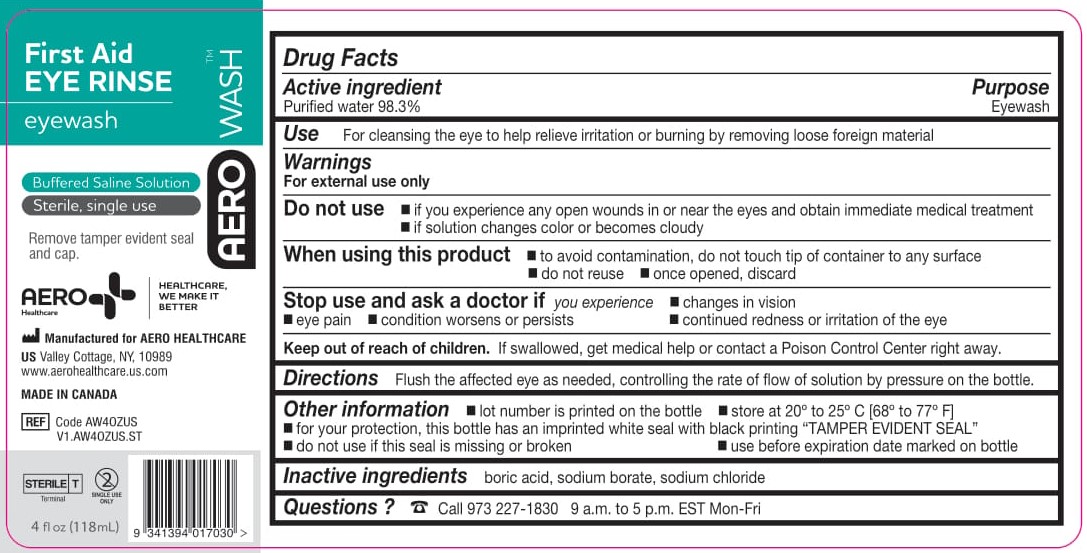
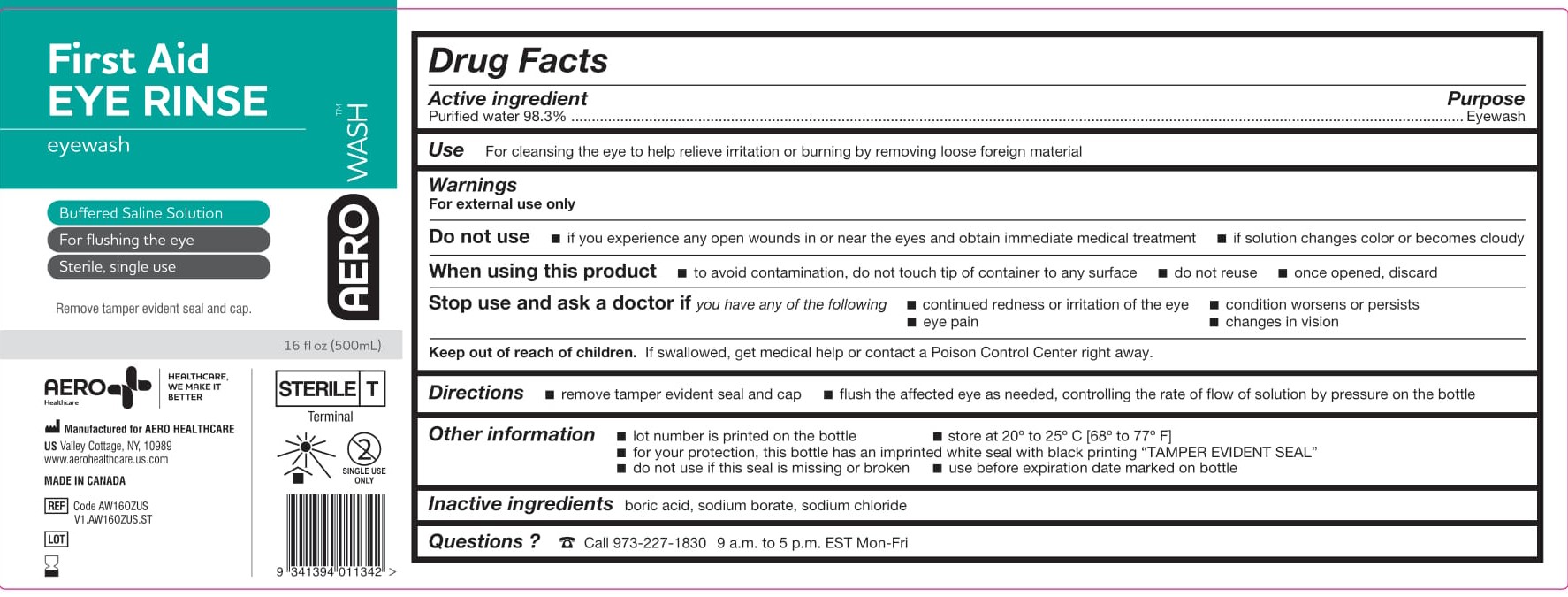
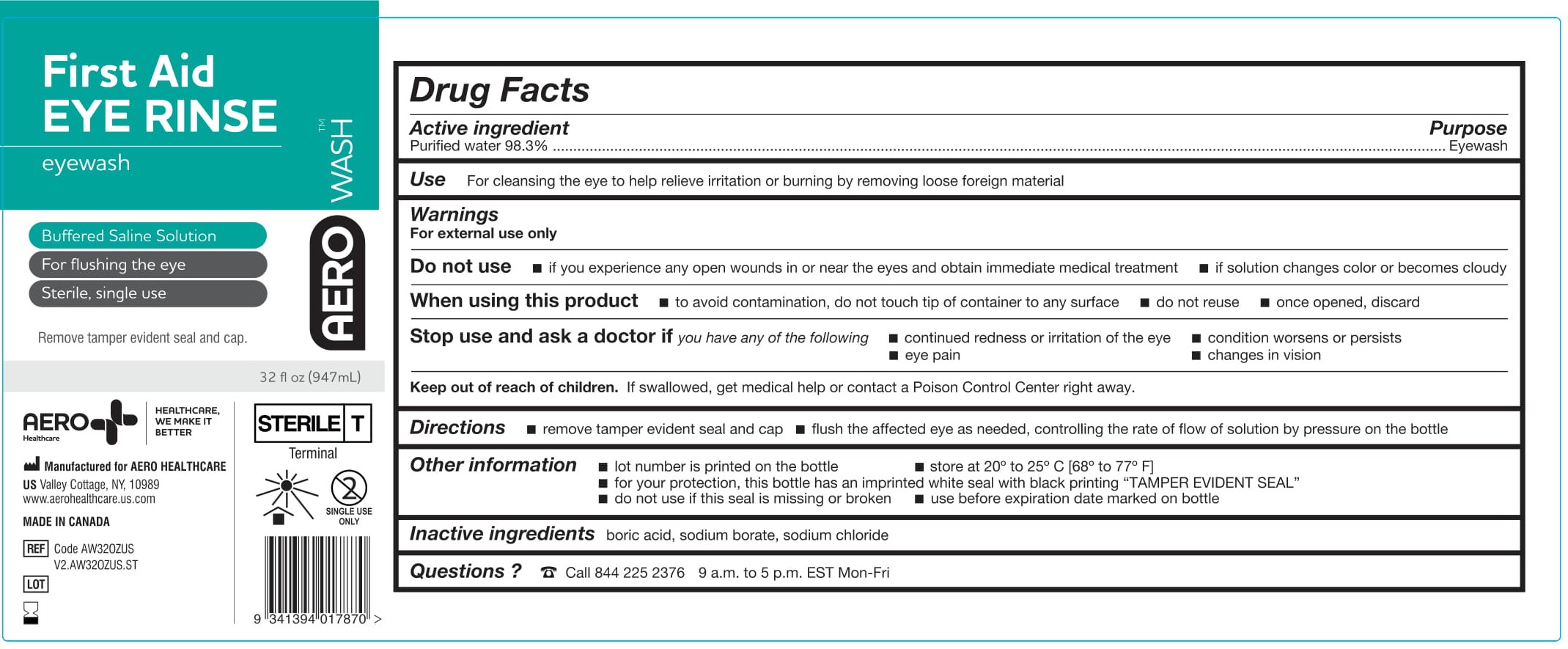
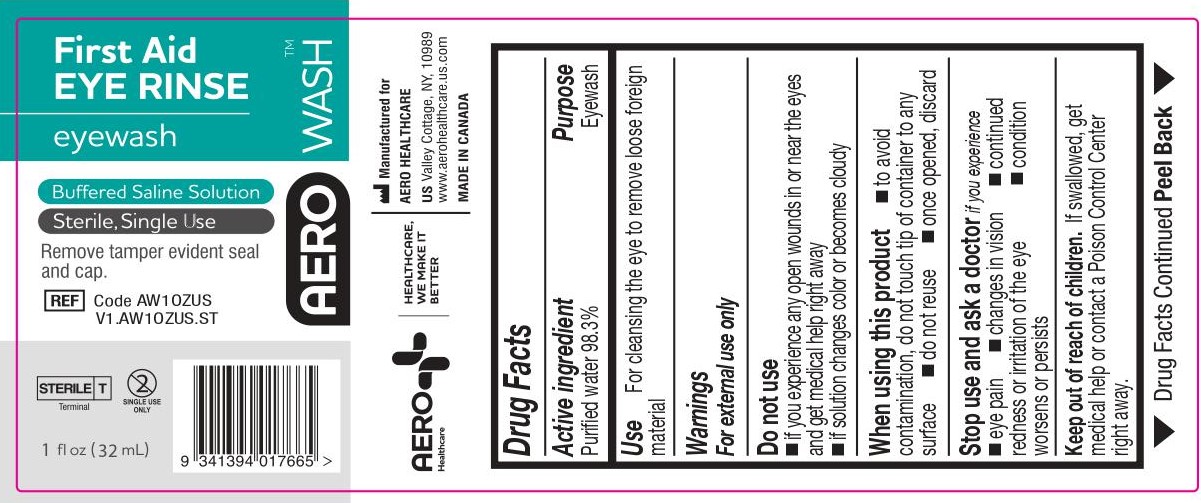
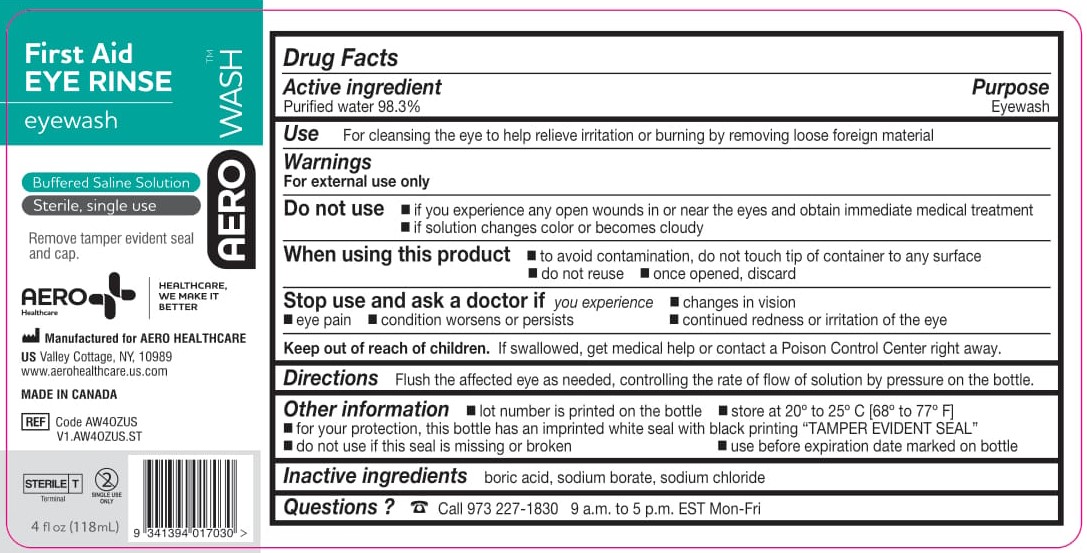
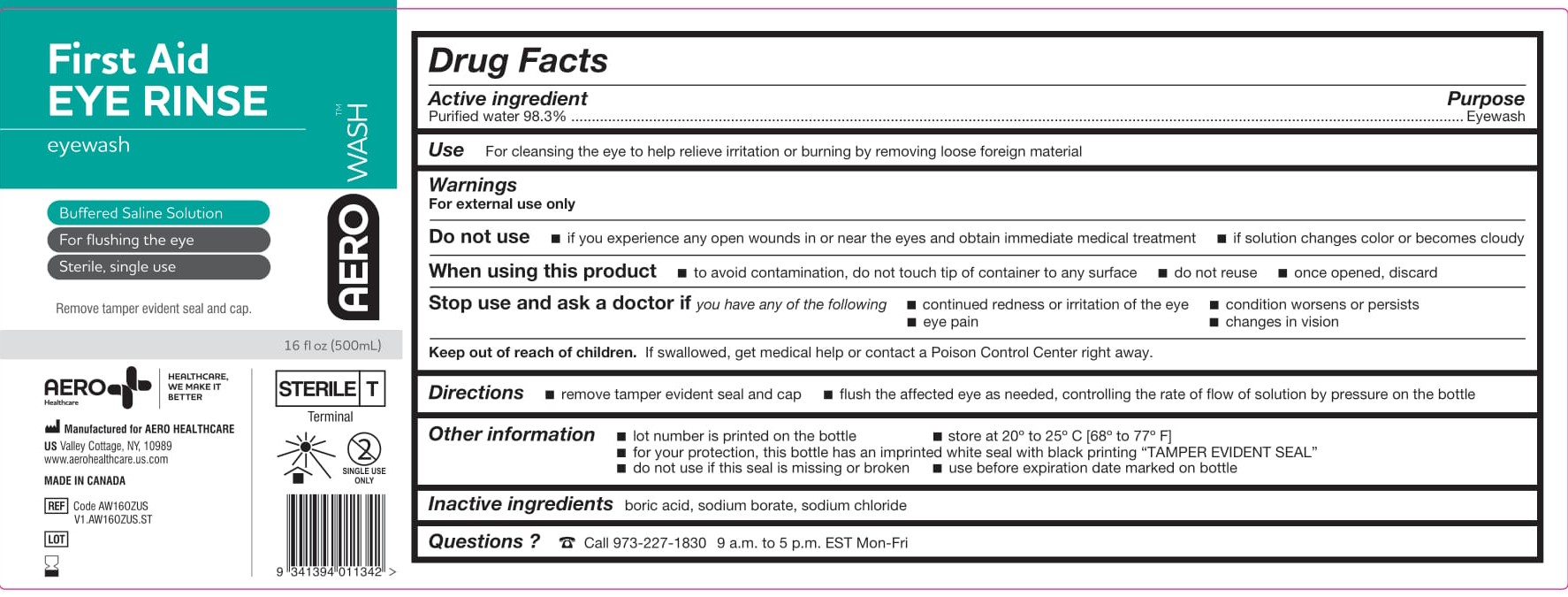
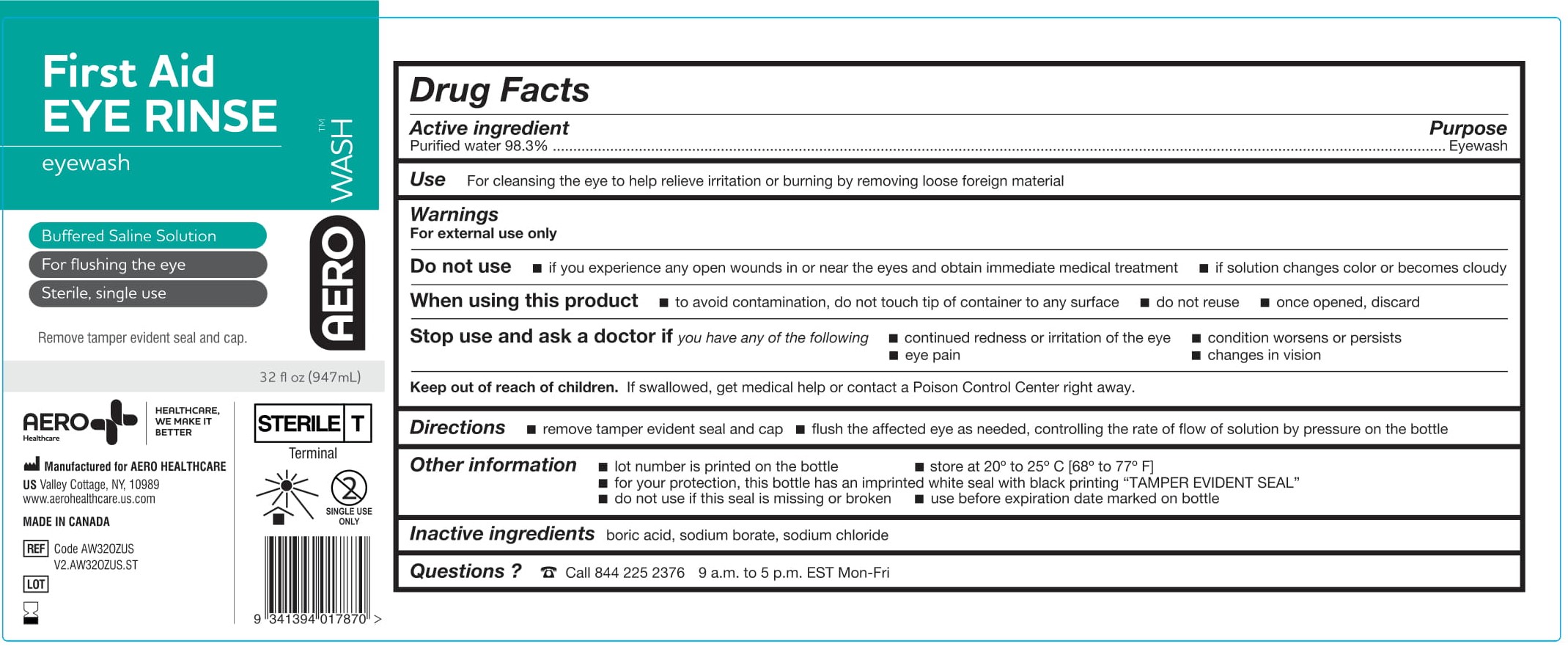
- ACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- Use
- KEEP OUT OF REACH OF CHILDREN
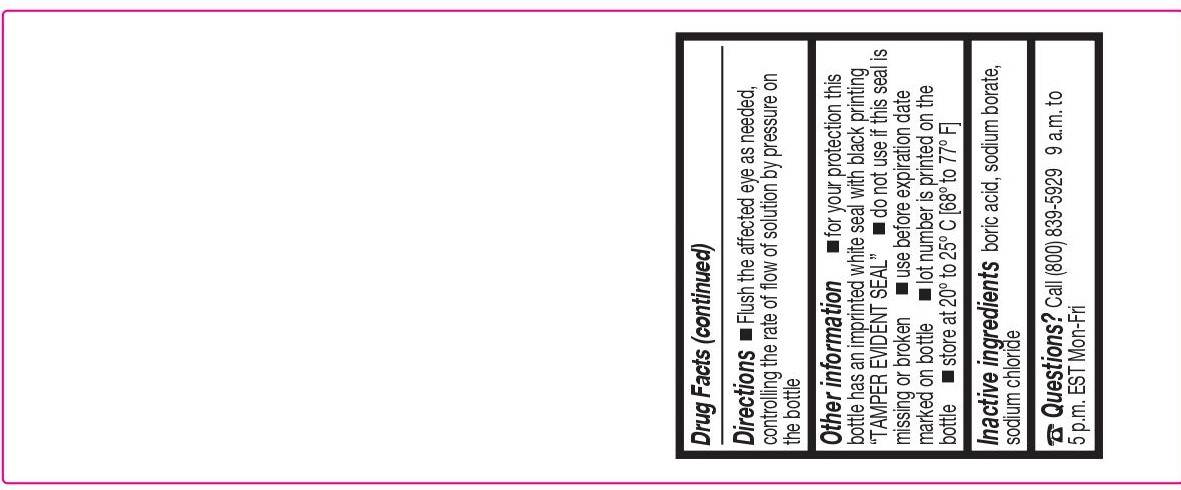
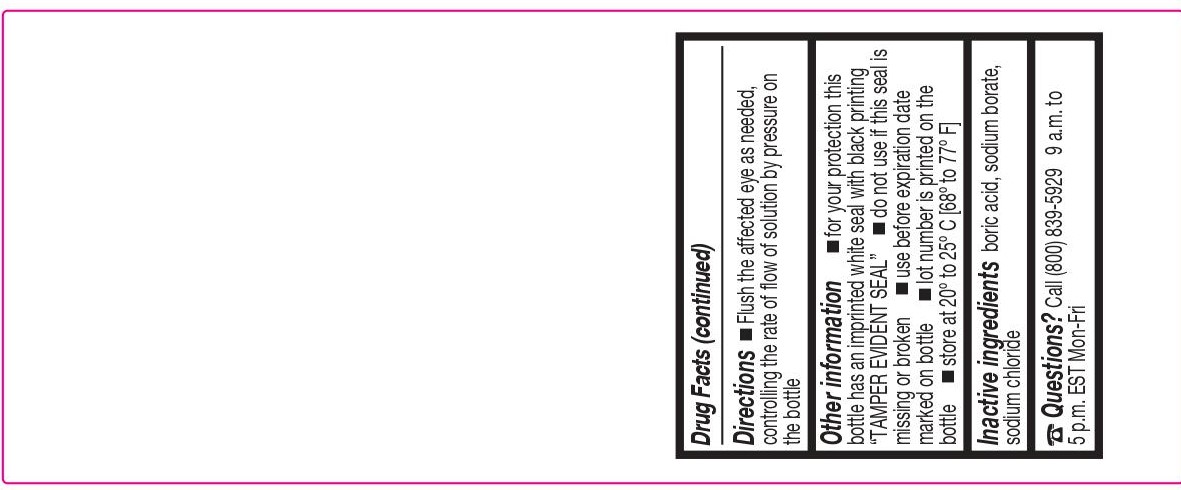
- DIRECTIONS
- OTHER INFORMATION
- INACTIVE INGREDIENTS
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 1 FL OZ (30 ML) LABEL
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 4 FL OZ (118 ML)
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 16 FL OZ (473 ML)
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 32 FL OZ (946 ML)
-
INGREDIENTS AND APPEARANCE
AEROWASH EYEWASH
eyewash solution liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:55305-115 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 0.983 mL in 1 mL Inactive Ingredients Ingredient Name Strength BORIC ACID (UNII: R57ZHV85D4) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM BORATE (UNII: 91MBZ8H3QO) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55305-115-10 30 mL in 1 BOTTLE, UNIT-DOSE; Type 0: Not a Combination Product 01/01/2020 2 NDC:55305-115-11 118 mL in 1 BOTTLE, UNIT-DOSE; Type 0: Not a Combination Product 01/01/2020 3 NDC:55305-115-12 473 mL in 1 BOTTLE, UNIT-DOSE; Type 0: Not a Combination Product 01/01/2020 4 NDC:55305-115-13 946 mL in 1 BOTTLE, UNIT-DOSE; Type 0: Not a Combination Product 01/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022305 01/01/2020 Labeler - Aero Healthcare US LLC (008186174) Registrant - Aero Healthcare US LLC (008186174)