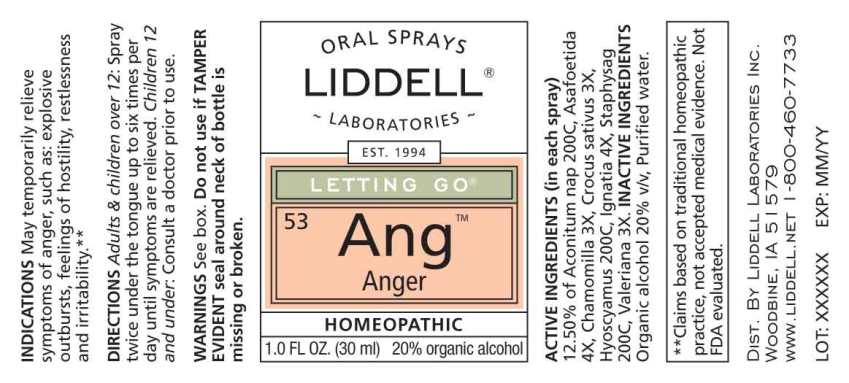
Label: ANGER- aconitum napellus, asafoetida, chamomilla, crocus sativus, hyoscyamus niger, ignatia amara, staphysagria, valeriana officinalis spray
- NDC Code(s): 50845-0269-1, 50845-0269-2
- Packager: Liddell Laboratories
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated December 1, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENTS:
- USES:
-
WARNINGS:
Do not use if you have ever had an allergic reaction to this product or any of its ingredients.
Stop use and ask a doctor if symptoms persist for more than 7 days, worsen, or if new symptoms occur.
Keep out of reach of children. In case of overdose, get medical help or call a Poison Control Center right away.
If pregnant or breast-feeding, ask a health professional before use.
Do not use if TAMPER EVIDENT seal around neck of bottle is missing or broken.
Store at room temperature.
- DIRECTIONS:
- INACTIVE INGREDIENTS:
- KEEP OUT OF REACH OF CHILDREN:
- USES:
- QUESTIONS:
- PACKAGE LABEL DISPLAY:
-
INGREDIENTS AND APPEARANCE
ANGER
aconitum napellus, asafoetida, chamomilla, crocus sativus, hyoscyamus niger, ignatia amara, staphysagria, valeriana officinalis sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50845-0269 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACONITUM NAPELLUS WHOLE (UNII: U0NQ8555JD) (ACONITUM NAPELLUS WHOLE - UNII:U0NQ8555JD) ACONITUM NAPELLUS WHOLE 200 [hp_C] in 1 mL FERULA ASSA-FOETIDA RESIN (UNII: W9FZA51AS1) (FERULA ASSA-FOETIDA RESIN - UNII:W9FZA51AS1) FERULA ASSA-FOETIDA RESIN 4 [hp_X] in 1 mL MATRICARIA CHAMOMILLA WHOLE (UNII: G0R4UBI2ZZ) (MATRICARIA CHAMOMILLA WHOLE - UNII:G0R4UBI2ZZ) MATRICARIA CHAMOMILLA WHOLE 3 [hp_X] in 1 mL SAFFRON (UNII: E849G4X5YJ) (SAFFRON - UNII:E849G4X5YJ) SAFFRON 3 [hp_X] in 1 mL HYOSCYAMUS NIGER (UNII: 4WRK2153H3) (HYOSCYAMUS NIGER - UNII:4WRK2153H3) HYOSCYAMUS NIGER 200 [hp_C] in 1 mL STRYCHNOS IGNATII SEED (UNII: 1NM3M2487K) (STRYCHNOS IGNATII SEED - UNII:1NM3M2487K) STRYCHNOS IGNATII SEED 4 [hp_X] in 1 mL DELPHINIUM STAPHISAGRIA SEED (UNII: 00543AP1JV) (DELPHINIUM STAPHISAGRIA SEED - UNII:00543AP1JV) DELPHINIUM STAPHISAGRIA SEED 200 [hp_C] in 1 mL VALERIAN (UNII: JWF5YAW3QW) (VALERIAN - UNII:JWF5YAW3QW) VALERIAN 3 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50845-0269-2 1 in 1 CARTON 02/23/2021 1 NDC:50845-0269-1 30 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 02/23/2021 Labeler - Liddell Laboratories (832264241) Registrant - Apotheca Company (844330915) Establishment Name Address ID/FEI Business Operations Apotheca Company 844330915 manufacture(50845-0269) , api manufacture(50845-0269) , label(50845-0269) , pack(50845-0269)