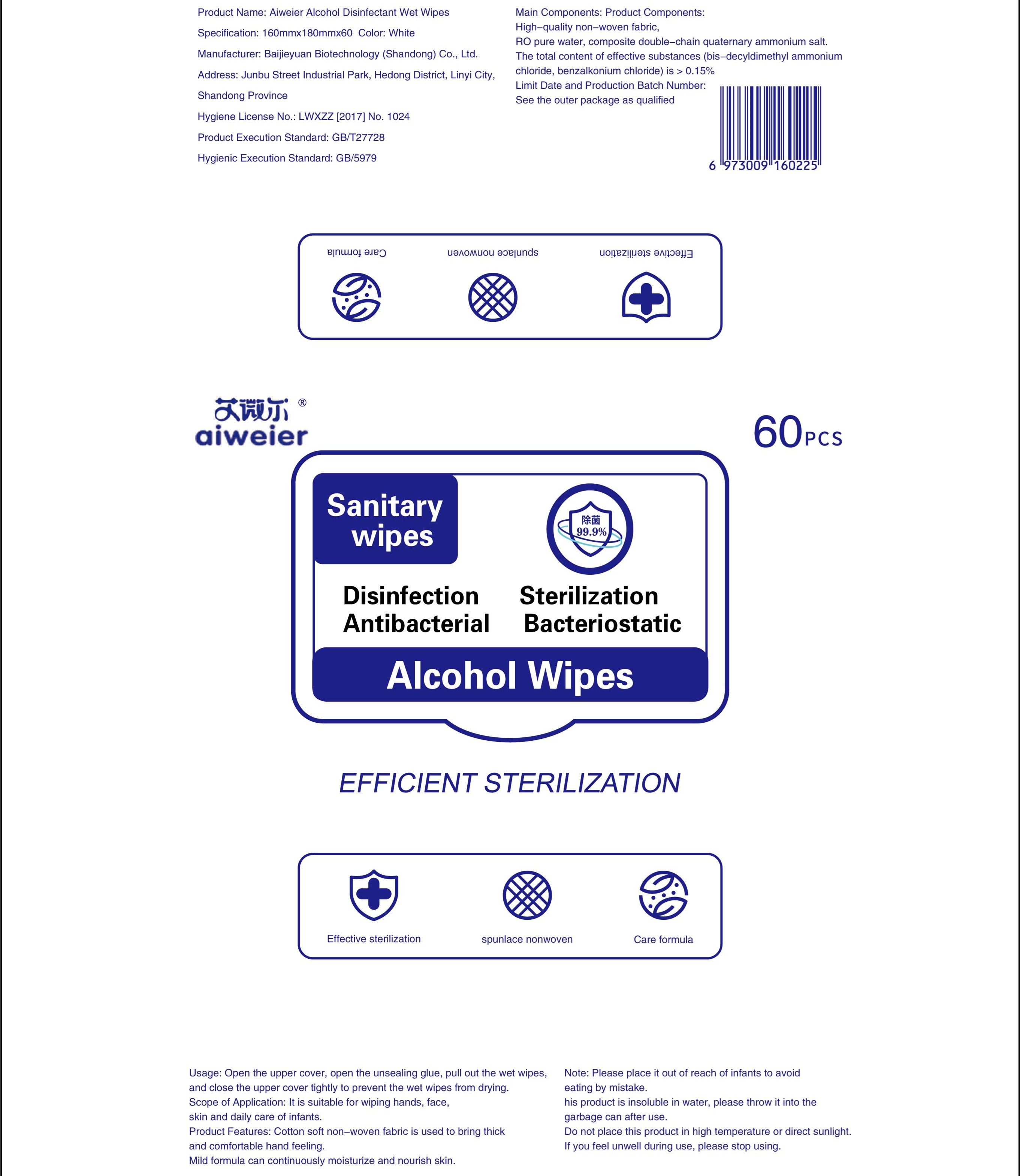
DISINFECTANT WET WIPES- wet wipes cloth
Baijieyuan Biotechnology (Shandong) Co., Ltd.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Open the upper cover, open the unsealing glue, pull out the wet wipes,and close the upper cover tightly to prevent the wet wipes from drying.
Bronopol
Benzethonium chloride
PhenoXyaethanolum
Benzalkonium chloride
1,3-Butanediol
Glycerol,Glucerine
Aloe Vera Extract
| DISINFECTANT WET WIPES
wet wipes cloth |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Baijieyuan Biotechnology (Shandong) Co., Ltd. (554531114) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Baijieyuan Biotechnology (Shandong) Co., Ltd. | 554531114 | manufacture(55490-001) | |
Revised: 12/2020
Document Id: b7816ed4-cdeb-a32f-e053-2a95a90a8ee9
Set id: a376d2bb-3562-7a99-e053-2a95a90aa0be
Version: 2
Effective Time: 20201228
Baijieyuan Biotechnology (Shandong) Co., Ltd.