AZITHROMYCIN DIHYDRATE- azithromycin dihydrate tablet, coated
Lannett Company, Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Azithromycin Film-Coated Tablets
IMPORTANT PRESCRIBING INFORMATION
April 2020
Subject: Temporary Importation of Azithromycin Tablets to Address Drug Shortage
Dear Healthcare Professional,
Due to the current critical shortage of Azithromycin tablet products in the United States (U.S.) market, HEC Pharm USA Inc. (HEC), in conjunction with Lannett Company, Inc. (Lannett), is coordinating with the U.S. Food and Drug Administration (FDA) to increase the availability of the drug. HEC has initiated temporary importation of non-FDA approved Azithromycin Film-Coated Tablets (500 mg) into the U.S. market. The Azithromycin Tablets are approved and marketed in Germany in accordance to the EU requirements. The product is manufactured in a facility approved by the FDA and packaged in an EU approved facility.
At this time, no other entity except HEC or its distributor Lannett is authorized by the FDA to import or distribute HEC’s Azithromycin Tablets in the United States. FDA has not approved HEC’s Azithromycin Tablets manufactured for the German market.
Effective immediately and during this temporary period, Lannett will distribute the presentations listed in below Table.
|
Product Name and Description |
Size |
NDC |
| Azithromycin Film-Coated Tablets 500 mg |
3 tabs/blister,1 blister/carton |
0527-2750-17 |
There are some key differences in the labeling between the U.S. marketed azithromycin and HEC’s imported Azithromycin Tablets. It is important to note the following:
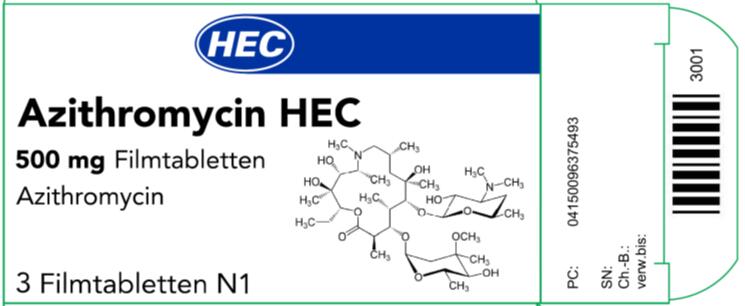
- The imported azithromycin product labeling contains German only text used and approved for marketing in Germany. An example image of this labeling is provided below. Each bundle (10 cartons per bundle) of imported azithromycin contains an English translated package leaflet and one copy of this DHCP Letter. The following German terminology and translated meaning are present on the imported azithromycin product labeling:
- The terminology “Ch. –B.” means: Batch Number/Lot Number
- The terminology “Verw. bis” means: Expiration date
- The terminology “SN” means: Serial number which is for the EU tracking system;
Example of the carton for the Azithromycin 500mg
- The imported product is labeled “Azithromycin HEC 500mg Filmtabletten” which means Azithromycin HEC 500 mg Film-coated Tablets, HEC is the Marketing Authorization (MA) Holder’s name which was allowed to be added to the product name in Germany.
- The imported azithromycin product blister packaging is not child-resistant. It is important to store the product out of reach of children.
- The barcode on the imported product label may not register with U.S. scanning systems. Institutions should manually input the product information into their systems to confirm that barcode systems do not provide incorrect information when the product is scanned. Alternative procedures should be followed to assure that the correct drug product is being used and administered to individual patients.
Azithromycin Tablet is available only by prescription in the U.S. Please refer to the FDA-approved package insert for the full prescribing information of Azithromycin 500 mg tablets at: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a2b0c06b-e93d-f5aa-e053-2995a90ac9aa
If you have any questions about the information contained in this letter or the use of the imported products, please contact Lannett Company, Inc. at (215) 333-9000, extension 4. This DHCP letter is posted at: http://www.lannett.com.
To place an order, please contact Lannett Company, Inc. for service by calling (215) 333-9000, extension 4.
To report product quality issues or to replace missing barcode stickers, please contact Lannett Company, Inc. for Service by calling 1-844-834-0530.
To report adverse events associated with these imported products, please call Lannett Company, Inc. at 1-844-834-0530. Adverse events or quality problems experienced with the use of this product may also be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax:
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
- Regular mail or Fax: Download form http://www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178.
We remain at your disposal to answer any questions you may have about our product; and provide more information if needed.
Sincerely,

Weiheng (Kevin) Kong
CEO & President
HEC Pharm USA Inc.
9000 State Road,
Philadelphia, PA, 19136, US
Phone: 267-348-3664
Email: ra_hec@hecpharm.com;
| AZITHROMYCIN DIHYDRATE
azithromycin dihydrate tablet, coated |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - Lannett Company, Inc. (002277481) |
| Registrant - Sunshine Lake Pharma Co., Ltd. (545391443) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Formula Pharmazeutische und chemische Entwicklungs GmbH | 313712622 | analysis(0527-2750) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| MARIFARM, d.o.o. | 360192929 | pack(0527-2750) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sunshine Lake Pharma Co., Ltd. | 545391443 | manufacture(0527-2750) | |