Label: COLESEVELAM HYDROCHLORIDE tablet, film coated
- NDC Code(s): 69452-158-25
- Packager: Bionpharma Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 14, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use COLESEVELAM HYDROCHLORIDE TABLETS safely and effectively. See full prescribing information for COLESEVELAM HYDROCHLORIDE TABLETS.
COLESEVELAM HYDROCHLORIDE tablets, for oral use
Initial U.S. Approval: 2000INDICATIONS AND USAGE
Colesevelam hydrochloride is a bile acid sequestrant indicated as an adjunct to diet and exercise to:
- reduce elevated low-density lipoprotein cholesterol (LDL-C) in adults with primary hyperlipidemia ( 1.1).
- reduce LDL-C levels in boys and post-menarchal girls, 10 years to 17 years of age, with heterozygous familial hypercholesterolemia (HeFH), unable to reach LDL-C target levels despite an adequate trial of diet and lifestyle modification ( 1.1).
Limitations of Use ( 1.3):
- Do not use for treatment of type 1 diabetes or for diabetic ketoacidosis.
- Not studied in Fredrickson Type I, III, IV, and V dyslipidemias.
DOSAGE AND ADMINISTRATION
- Obtain lipid parameters, including serum triglyceride (TG) levels, before starting colesevelam hydrochloride tablets ( 2.1)
- The recommended dosage for adults and for boys and post-menarchal girls aged 10 years to 17 years with primary hyperlipidemia is 3.75 grams daily. Colesevelam hydrochloride tablets should be taken as follows ( 2.2, 2.4):
Take 6 tablets once daily or 3 tablets twice daily with a meal and liquid.
DOSAGE FORMS AND STRENGTHS
- Tablets: 625 mg ( 3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Hypertriglyceridemia and Pancreatitis: Colesevelam hydrochloride can increase TG. Hypertriglyceridemia can cause acute pancreatitis. Monitor lipids, including TG. Instruct patients to discontinue colesevelam hydrochloride and seek prompt medical attention if the symptoms of acute pancreatitis occur ( 5.1).
- Gastrointestinal Obstruction: Cases of bowel obstruction have occurred. Colesevelam hydrochloride is not recommended in patients with gastroparesis, other gastrointestinal motility disorders, and in those who have had major gastrointestinal tract surgery and who may be at risk for bowel obstruction ( 5.2).
- Vitamin K or Fat-Soluble Vitamin Deficiencies: Colesevelam hydrochloride may decrease absorption of fat-soluble vitamins. Patients with a susceptibility to deficiencies of vitamin K (e.g., patients on warfarin, patients with malabsorption syndromes) or other fat-soluble vitamins may be at increased risk. Patients on oral vitamin supplementation should take their vitamins at least 4 hours prior to colesevelam hydrochloride ( 5.3).
- Drug Interactions: Due to the potential for decreased absorption of other drugs that have not been tested for interaction, consider administering at least 4 hours prior to colesevelam hydrochloride ( 5.4, 7, 12.3).
ADVERSE REACTIONS
In clinical trials, the most common (incidence ≥ 2% and greater than placebo) adverse reactions with colesevelam hydrochloride included constipation, dyspepsia, and nausea ( 6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Bionpharma Inc. at 1-888-235-BION or 1-888-235-2466 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Concomitant use with colesevelam hydrochloride may decrease the exposure of the following drugs: Drugs with a narrow therapeutic index (e.g., cyclosporine), phenytoin, thyroid hormone replacement therapy, warfarin, oral contraceptives containing ethinyl estradiol and norethindrone, olmesartan medoxomil, and sulfonylureas (glimepiride, glipizide, glyburide). Administer these drugs 4 hours prior to colesevelam hydrochloride. For patients on warfarin, monitor International Normalized Ratio (INR) frequently during initiation then periodically ( 7.1).
Concomitant use with colesevelam hydrochloride may increase the exposure of the following drugs: Metformin extended release. Monitor patients' glycemic control ( 7.2).
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 11/2021
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Primary Hyperlipidemia
1.3 Limitations of Use
2 DOSAGE AND ADMINISTRATION
2.1 Testing Prior to Initiation of Colesevelam Hydrochloride Tablets
2.2 Recommended Dosage in Primary Hyperlipidemia
2.3 Important Dosing Information for Primary Hyperlipidemia
2.4 Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypertriglyceridemia and Pancreatitis
5.2 Gastrointestinal Obstruction
5.3 Vitamin K or Fat-Soluble Vitamin Deficiencies
5.4 Drug Interactions
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
6.2 Post-marketing Experience
7 DRUG INTERACTIONS
7.1 Colesevelam Hydrochloride Drug Interactions that Decrease the Exposure of the Concomitant Medication
7.2 Colesevelam Hydrochloride Drug Interactions that Increase the Exposure of the Concomitant Medication
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Primary Hyperlipidemia
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Primary Hyperlipidemia
Colesevelam hydrochloride tablets are indicated as an adjunct to diet and exercise to reduce elevated low-density lipoprotein cholesterol (LDL-C) in adults with primary hyperlipidemia.
Colesevelam hydrochloride tablets are indicated to reduce LDL-C levels in boys and post-menarchal girls, 10 years to 17 years of age, with heterozygous familial hypercholesterolemia (HeFH) who are unable to reach LDL-C target levels despite an adequate trial of dietary therapy and lifestyle modification.
-
2 DOSAGE AND ADMINISTRATION
2.1 Testing Prior to Initiation of Colesevelam Hydrochloride Tablets
Obtain lipid parameters, including triglyceride (TG) levels, before starting colesevelam hydrochloride tablets. Colesevelam hydrochloride is contraindicated in patients with TG levels > 500 mg/dL [see Contraindications (4) and Warnings and Precautions (5.1)] .
2.2 Recommended Dosage in Primary Hyperlipidemia
The recommended dosage of colesevelam hydrochloride for adults and for boys and post-menarchal girls aged 10 years to 17 years with primary hyperlipidemia is 3.75 grams daily. Colesevelam hydrochloride tablets should be taken as follows:
2.3 Important Dosing Information for Primary Hyperlipidemia
Colesevelam hydrochloride can be dosed at the same time as a statin, or colesevelam hydrochloride and the statin can be dosed apart . Monitor lipid levels within 4 weeks to 6 weeks after initiation of colesevelam hydrochloride.
2.4 Administration Instructions
Take colesevelam hydrochloride tablets with a meal and liquid. For patients with difficulty swallowing tablets, use colesevelam hydrochloride for oral suspension [see Warnings and Precautions (5.2)].
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Colesevelam hydrochloride is contraindicated in patients with:
- Serum TG concentrations > 500 mg/dL [see Warnings and Precautions (5.1)]
- History of hypertriglyceridemia-induced pancreatitis [see Warnings and Precautions (5.1)]
- A history of bowel obstruction [see Warnings and Precautions (5.2)]
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypertriglyceridemia and Pancreatitis
Colesevelam hydrochloride, like other bile acid sequestrants, can increase serum TG concentrations. Hypertriglyceridemia can cause acute pancreatitis.
Colesevelam hydrochloride had effects on serum TG (median increase 5% compared to placebo) in trials of patients with primary hyperlipidemia.
Obtain lipid parameters, including TG levels, before starting colesevelam hydrochloride and periodically thereafter. Colesevelam hydrochloride is contraindicated in patients with TG levels > 500 mg/dL or patients with a history of hypertriglyceridemia-induced pancreatitis [see Contraindications (4)] . Patients with TG levels greater than 300 mg/dL could have greater increases in serum TG levels with colesevelam hydrochloride and may require additional TG monitoring. Instruct patients to discontinue colesevelam hydrochloride and seek prompt medical attention if the symptoms of acute pancreatitis occur (e.g., severe abdominal pain with or without nausea and vomiting). Discontinue colesevelam hydrochloride if TG levels exceed 500 mg/dL [see Adverse Reactions (6.1)] .
5.2 Gastrointestinal Obstruction
Postmarketing cases of bowel obstruction have occurred with colesevelam hydrochloride [see Adverse Reactions (6.2)] . Because of its constipating effects, colesevelam hydrochloride is not recommended in patients with gastroparesis, other gastrointestinal motility disorders, and in those who have had major gastrointestinal tract surgery and who may be at risk for bowel obstruction. Colesevelam hydrochloride is contraindicated in patients with a history of bowel obstruction [see Contraindications (4)] . Instruct patients to promptly discontinue colesevelam hydrochloride and seek medical attention if severe abdominal pain or severe constipation occurs.
Because of the tablet size, colesevelam hydrochloride tablets can cause dysphagia or esophageal obstruction. For patients with difficulty swallowing tablets, use colesevelam hydrochloride for oral suspension.
5.3 Vitamin K or Fat-Soluble Vitamin Deficiencies
Colesevelam hydrochloride may decrease the absorption of fat-soluble vitamins A, D, E, and K. Patients with a susceptibility to deficiencies of vitamin K (e.g., patients on warfarin, patients with malabsorption syndromes) or other fat-soluble vitamins may be at increased risk when taking colesevelam hydrochloride.
Patients on oral vitamin supplementation should take their vitamins at least 4 hours prior to colesevelam hydrochloride [see Drug Interactions (7.1)].
5.4 Drug Interactions
Colesevelam hydrochloride reduces gastrointestinal absorption of some drugs. Administer drugs with a known interaction at least 4 hours prior to colesevelam hydrochloride [see Drug Interactions (7)] .
Due to the potential for decreased absorption of other drugs that have not been tested for interaction, especially those with a narrow therapeutic index, consider administering at least 4 hours prior to colesevelam hydrochloride [see Clinical Pharmacology (12.3)].
-
6 ADVERSE REACTIONS
The following important adverse reactions are described below and elsewhere in the labeling:
- Hypertriglyceridemia and Pancreatitis [see Warnings and Precautions (5.1)]
- Gastrointestinal Obstruction [see Warnings and Precautions (5.2)]
- Vitamin K or Fat-Soluble Vitamin Deficiencies [see Warnings and Precautions (5.3)]
6.1 Clinical Studies Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in clinical studies of another drug and may not reflect the rates observed in practice.
Primary Hyperlipidemia
In 7 double-blind, placebo-controlled clinical trials, 807 patients with primary hyperlipidemia (age range 18 years to 86 years, 50% women, 90% Caucasians, 7% Blacks, 2% Hispanics, 1% Asians) and elevated LDL-C were treated with colesevelam hydrochloride 1.5 g/day to 4.5 g/day from 4 weeks to 24 weeks (total exposure 199 patient-years).
Table 1 Clinical Studies of Colesevelam Hydrochloride for Primary Hyperlipidemia: Adverse Reactions Reported in ≥ 2% of Patients and More Commonly than in Placebo Colesevelam Hydrochloride
N = 807Placebo
N = 258Constipation 11% 7% Dyspepsia 8.3% 3.5% Nausea 4.2% 3.9% Accidental injury 3.7% 2.7% Asthenia 3.6% 1.9% Pharyngitis 3.2% 1.9% Flu syndrome 3.2% 3.1% Rhinitis 3.2% 3.1% Myalgia 2.1% 0.4% Pediatric Patients 10 Years to 17 Years of Age
In an 8-week double-blind, placebo-controlled study, boys and post-menarchal girls, 10 years to 17 years of age, with HeFH (n = 194), were treated with colesevelam hydrochloride tablets (1.9 g to 3.8 g, daily) or placebo tablets.
Table 2 Clinical Study of Colesevelam Hydrochloride for Primary Hyperlipidemia in HeFH Pediatric Patients: Adverse Reactions Reported in ≥ 2% of Patients and More Commonly than in Placebo Colesevelam Hydrochloride
N = 129Placebo
N = 65Nasopharyngitis 6.2% 4.6% Headache 3.9% 3.1% Fatigue 3.9% 1.5% Creatine Phosphokinase Increase 2.3% 0% Rhinitis 2.3% 0% Vomiting 2.3% 1.5% The reported adverse reactions during the additional 18-week open-label treatment period with colesevelam hydrochloride 3.8 g per day were similar to those during the double-blind period and included headache (7.6%), nasopharyngitis (5.4%), upper respiratory tract infection (4.9%), influenza (3.8%), and nausea (3.8%).
6.2 Post-marketing Experience
The following additional adverse reactions have been identified during post-approval use of colesevelam hydrochloride. Because these reactions are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Adverse Reactions Resulting from Drug Interactions [see Drug Interactions (7)]: Increased seizure activity or decreased phenytoin levels in patients receiving phenytoin, reduced International Normalized Ratio (INR) in patients receiving warfarin therapy, and elevated thyroid-stimulating hormone (TSH) in patients receiving thyroid hormone replacement therapy
-
7 DRUG INTERACTIONS
7.1 Colesevelam Hydrochloride Drug Interactions that Decrease the Exposure of the Concomitant Medication
Table 4 includes a list of drugs that decrease exposure of the concomitant medication when administered concomitantly with colesevelam hydrochloride and instructions for preventing or managing them.
Table 4 Colesevelam Hydrochloride Drug Interactions that Decrease the Exposure of the Concomitant Medication Drugs with a Narrow Therapeutic Index Clinical Impact: Concomitant use with colesevelam hydrochloride may decrease the exposure of the narrow therapeutic index drug. In vivo drug interactions studies showed a decrease in exposure of cyclosporine when co-administered with colesevelam hydrochloride [see Clinical Pharmacology (12.3)]. Intervention: Administer the narrow therapeutic index drug at least 4 hours prior to colesevelam hydrochloride. Monitor drug levels when appropriate. Examples: Cyclosporine Phenytoin Clinical Impact: There have been postmarketing reports of increased seizure activity or decreased phenytoin levels in patients receiving phenytoin [see Adverse Reactions (6.2)]. Intervention: Administer phenytoin 4 hours prior to colesevelam hydrochloride. Thyroid Hormone Replacement Therapy Clinical Impact: In vivo drug interactions studies showed a decrease in exposure of levothyroxine when co-administered with colesevelam hydrochloride [see Clinical Pharmacology (12.3)] . There have been postmarketing reports of elevated thyroid-stimulating hormone (TSH) in patients receiving thyroid hormone replacement therapy [see Adverse Reactions (6.2)] . Intervention: Administer thyroid hormone replacement therapy 4 hours prior to colesevelam hydrochloride. Warfarin Clinical Impact: There have been postmarketing reports of reduced INR in patients receiving warfarin therapy [see Adverse Reactions (6.2)]. Intervention: Monitor INR frequently during colesevelam hydrochloride initiation then periodically thereafter. Oral Contraceptives Containing Ethinyl Estradiol and Norethindrone Clinical Impact: In vivo drug interactions studies showed a decrease in exposure of ethinyl estradiol and norethindrone when co-administered with colesevelam hydrochloride [see Clinical Pharmacology (12.3)]. Intervention: Administer oral contraceptives containing ethinyl estradiol and norethindrone 4 hours prior to colesevelam hydrochloride. Olmesartan Medoxomil Clinical Impact: In vivo drug interactions studies showed a decrease in olmesartan medoxomil when co-administered with colesevelam hydrochloride [see Clinical Pharmacology (12.3)]. Intervention: Administer olmesartan medoxomil 4 hours prior to colesevelam hydrochloride. Sulfonylureas Clinical Impact: In vivo drug interactions studies showed a decrease in sulfonylureas when co-administered with colesevelam hydrochloride [see Clinical Pharmacology (12.3)]. Intervention: Administer sulfonylureas 4 hours prior to colesevelam hydrochloride. Examples: Glimepiride, glipizide, and glyburide Oral Vitamin Supplements Clinical Impact: Colesevelam hydrochloride may decrease the absorption of fat-soluble vitamins A, D, E, and K [see Warnings and Precautions (5.3)]. Intervention: Patients on oral vitamin supplementation should take their vitamins at least 4 hours prior to colesevelam hydrochloride. 7.2 Colesevelam Hydrochloride Drug Interactions that Increase the Exposure of the Concomitant Medication
Table 5 Colesevelam Hydrochloride Drug Interactions that Increase the Exposure of the Concomitant Medication Metformin Extended-Release (ER) Clinical Impact: In vivo drug interactions studies showed an increase in metformin extended release (ER) when co-administered with colesevelam hydrochloride [see Clinical Pharmacology (12.3)]. Intervention: Monitor patients' glycemic control. -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Colesevelam hydrochloride is not absorbed systemically following oral administration, and maternal use is not expected to result in fetal exposure to the drug. Limited available data on the use of colesevelam hydrochloride are insufficient to determine a drug-associated risk of major congenital malformations or miscarriage. In animal reproduction studies, no evidence of either maternal or fetal toxicity was found in rats or rabbits exposed to colesevelam hydrochloride during the period of fetal organogenesis at 8 and 5 times, respectively, the maximum recommended human dose (MRHD) of 3.75 g/day, based on body surface area (mg/m 2). No adverse effects on offspring survival and development were observed in rats administered 5 times the MRHD (see Data). Colesevelam hydrochloride may decrease the absorption of fat-soluble vitamins [see Warnings and Precautions (5.3)]. There are no data available on the effect of colesevelam hydrochloride on the absorption of fat-soluble vitamins in pregnant women. If the patient becomes pregnant while taking colesevelam hydrochloride, the patient should be advised of the lack of known clinical benefit with continued use during pregnancy.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the US general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Human Data
There are no adequate and well-controlled studies of colesevelam hydrochloride use in pregnant women. In the postmarketing setting there have been infrequent reports of pregnancy with use of colesevelam hydrochloride and a causal association with congenital anomalies has not been established.
Animal Data
In pregnant rats given dietary doses of 0.3 g/kg/day, 1 g/kg/day, 3 g/kg/day colesevelam hydrochloride from gestation days 7 through 17, no teratogenic effects were observed. Exposures at 3 g/kg/day were 8 times the human exposure at 3.75 g/day MRHD, based on body surface area (mg/m 2).
In pregnant rabbits given oral gavage doses of 0.1 g/kg/day, 0.5 g/kg/day, 1 g/kg/day colesevelam hydrochloride from gestation days 6 through 18, no teratogenic effects were observed. Exposures at 1 g/kg/day were 5 times the human exposure at 3.75 g/day MRHD, based on body surface area (mg/m 2).
In pregnant rats given oral gavage doses of 0.1 g/kg/day, 0.3 g/kg/day, 1 g/kg/day colesevelam hydrochloride from gestation day 6 through lactation day 21 (weaning), no adverse effects on survival and development were observed. Exposures at 1 g/kg/day were 5 times the human exposure at 3.75 g/day MRHD, based on body surface area (mg/m 2).
8.3 Females and Males of Reproductive Potential
Contraception
Use of colesevelam hydrochloride may reduce the efficacy of oral contraceptives. Advise patients to take oral contraceptives at least 4 hours prior to taking colesevelam hydrochloride [see Drug Interactions (7)].
8.4 Pediatric Use
Primary Hyperlipidemia
The safety and effectiveness of colesevelam hydrochloride to reduce LDL-C levels in boys and post-menarchal girls 10 years to 17 years of age with HeFH who are unable to reach LDL-C target levels despite an adequate trial of dietary therapy and lifestyle modification have been established. Use of colesevelam hydrochloride for this indication is supported by a study in 129 colesevelam hydrochloride-treated pediatric patients aged 10 years to 17 years with HeFH [see Clinical Studies (14.1)] . Adverse reactions commonly observed in pediatric patients compared to placebo, but not in adults, included headache (3.9%), creatine phosphokinase increase (2.3%), and vomiting (2.3%) [see Adverse Reactions (6.1)] . There were no significant effects on fat-soluble vitamin levels or clotting factors in the adolescent boys or girls relative to placebo. Due to colesevelam hydrochloride tablet size, colesevelam hydrochloride for oral suspension is recommended for use in the pediatric population [see Dosage and Administration (2.2, 2.4)]. The safety and effectiveness of colesevelam hydrochloride in pediatric patients with HeFH less than 10 years of age or in premenarchal females have not been established.
8.5 Geriatric Use
Primary Hyperlipidemia
Of the 1,350 patients enrolled in the hyperlipidemia clinical studies, 349 (26%) were ≥ 65 years old, and 58 (4%) were ≥ 75 years old. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
- 10 OVERDOSAGE
-
11 DESCRIPTION
Colesevelam hydrochloride is a non-absorbed, polymeric, lipid-lowering agent for oral administration. Colesevelam hydrochloride is a high-capacity bile acid-binding molecule.
Colesevelam hydrochloride is poly(allylamine hydrochloride) cross-linked with epichlorohydrin and alkylated with 1-bromodecane and (6-bromohexyl)-trimethylammonium bromide. The chemical name (IUPAC) of colesevelam hydrochloride is allylamine polymer with 1-chloro-2,3-epoxypropane, [6‑(allylamino)-hexyl]trimethylammonium chloride and N-allyldecylamine, hydrochloride. The chemical structure of colesevelam hydrochloride is represented by the following formula:
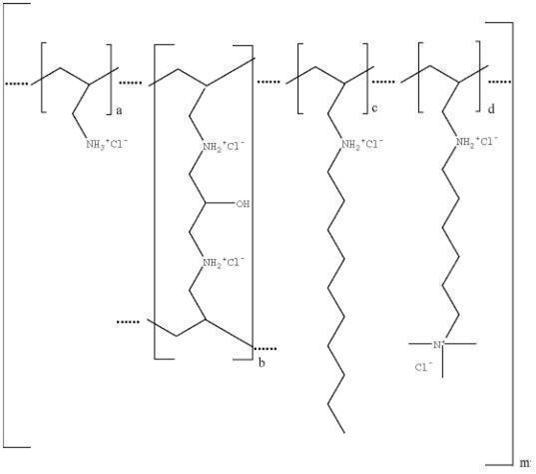
wherein (a) represents allyl amine monomer units that have not been alkylated by either of the 1‑bromodecane or (6-bromohexyl)-trimethylammonium bromide alkylating agents or cross-linked by epichlorohydrin; (b) represents allyl amine units that have undergone cross-linking with epichlorohydrin; (c) represents allyl amine units that have been alkylated with a decyl group; (d) represents allyl amine units that have been alkylated with a (6-trimethylammonium) hexyl group, and m represents a number ≥ 100 to indicate an extended polymer network. A small amount of the amines are dialkylated and are not depicted in the formula above. No regular order of the groups is implied by the structure; cross-linking and alkylation are expected to occur randomly along the polymer chains. A large amount of the amines are protonated. The polymer is depicted in the hydrochloride form; a small amount of the halides are bromide. Colesevelam hydrochloride is hydrophilic and insoluble in water.
Colesevelam hydrochloride tablets are white to off-white, oval-shaped, film-coated tablets, printed with “COL” on one side with black ink containing 625 mg colesevelam hydrochloride. In addition, each tablet contains the following inactive ingredients: magnesium stearate, microcrystalline cellulose, silicon dioxide. The tablets are imprinted using a water-soluble black ink (ferrosoferric oxide, hypromellose, and propylene glycol). The coating material contains polyethylene glycol, polysorbate 80 and talc (approximately 5 calories per 6 tablets).
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Primary Hyperlipidemia: Colesevelam hydrochloride, the active pharmaceutical ingredient in colesevelam hydrochloride tablets, is a non-absorbed, lipid-lowering polymer that binds bile acids in the intestine, impeding their reabsorption. As the bile acid pool becomes depleted, the hepatic enzyme, cholesterol 7-α-hydroxylase, is upregulated, which increases the conversion of cholesterol to bile acids. This causes an increased demand for cholesterol in the liver cells, resulting in the dual effect of increasing transcription and activity of the cholesterol biosynthetic enzyme, HMG-CoA reductase, and increasing the number of hepatic LDL receptors. These compensatory effects result in increased clearance of LDL-C from the blood, resulting in decreased serum LDL-C levels. Serum TG levels may increase or remain unchanged.
12.2 Pharmacodynamics
A maximum therapeutic response to the lipid-lowering effects of colesevelam hydrochloride was achieved within 2 weeks and was maintained during long-term therapy.
12.3 Pharmacokinetics
Absorption
Colesevelam hydrochloride is a hydrophilic, water-insoluble polymer that is not hydrolyzed by digestive enzymes and is not absorbed.
Distribution
Colesevelam hydrochloride is not absorbed, and therefore, its distribution is limited to the gastrointestinal tract.
Elimination
Metabolism
Colesevelam hydrochloride is not metabolized systemically and does not interfere with systemic drug-metabolizing enzymes such as cytochrome P450.
Excretion
In 16 healthy volunteers, an average of 0.05% of administered radioactivity from a single 14C-labeled colesevelam hydrochloride dose was excreted in the urine.
Drug Interaction Studies
Drug interactions between colesevelam and concomitantly administered drugs were screened through in vitro studies and confirmed in in vivo studies. In vitro studies demonstrated that cephalexin, metformin, and ciprofloxacin had negligible binding to colesevelam hydrochloride. Therefore, an in vivo pharmacokinetic interaction of colesevelam hydrochloride with these drugs is unlikely. Colesevelam hydrochloride was found to have no significant effect on the bioavailability of aspirin, atenolol, digoxin, enalapril, fenofibrate, lovastatin, metoprolol, phenytoin, pioglitazone, quinidine, rosiglitazone, sitagliptin, valproic acid, and warfarin. The results of additional in vivo drug interactions of colesevelam hydrochloride are presented in Table 6.
Table 6 Mean Change in Drug Exposure (AUC 0-∞ and C max) when Administered with Colesevelam Hydrochloride (3.75 g) * Drug Dose Co-administered 1 hr prior to Colesevelam Hydrochloride 4 hrs prior to Colesevelam Hydrochloride AUC 0-∞ C max AUC 0-∞ C max AUC 0-∞ C max N/A Not Available Cyclosporine 200 mg -34% -44% N/A N/A N/A N/A Ethinyl Estradiol † 0.035 mg -24% -24% -18% -1% -12% 0% Glimepiride 4 mg -18% -8% N/A N/A -6% 3% Glipizide 20 mg -12% -13% N/A N/A -4% 0% Glyburide 3 mg -32% -47% -20% -15% -7% 4% Levothyroxine 600 mcg -22% -33% 6% -2% 1% 8% Metformin ER 1,500 mg 44% 8% N/A N/A N/A N/A Norethindrone † 1 mg -1% -20% 5% -3% 6% 7% Olmesartan Medoxomil 40 mg -39% -28% N/A N/A -15% -4% Repaglinide 2 mg -7% -19% -6% -1% N/A N/A Verapamil Sustained Release 240 mg -31% -11% N/A N/A N/A N/A NA - not available
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
A 104-week carcinogenicity study with colesevelam hydrochloride was conducted in CD-1 mice, at oral dietary doses up to 3 g/kg/day. This dose was approximately 50 times the maximum recommended human dose of 4.5 g/day, based on body weight, mg/kg. There were no significant drug-induced tumor findings in male or female mice. In a 104-week carcinogenicity study with colesevelam hydrochloride in Harlan Sprague-Dawley rats, a statistically significant increase in the incidence of pancreatic acinar cell adenoma was seen in male rats at doses > 1.2 g/kg/day (approximately 20 times the maximum human dose, based on body weight, mg/kg) (trend test only). A statistically significant increase in thyroid C-cell adenoma was seen in female rats at 2.4 g/kg/day (approximately 40 times the maximum human dose, based on body weight, mg/kg).
Mutagenesis
Colesevelam hydrochloride and 4 degradants present in the drug substance have been evaluated for mutagenicity in the Ames test and a mammalian chromosomal aberration test. The 4 degradants and an extract of the parent compound did not exhibit genetic toxicity in an in vitro bacterial mutagenesis assay in S. typhimurium and E. coli (Ames assay) with or without rat liver metabolic activation. An extract of the parent compound was positive in the Chinese Hamster Ovary (CHO) cell chromosomal aberration assay in the presence of metabolic activation and negative in the absence of metabolic activation. The results of the CHO cell chromosomal aberration assay with 2 of the 4 degradants, decylamine HCl and aminohexyltrimethyl ammonium chloride HCl, were equivocal in the absence of metabolic activation and negative in the presence of metabolic activation. The other 2 degradants, didecylamine HCl and 6-decylamino-hexyltrimethyl ammonium chloride HCl, were negative in the presence and absence of metabolic activation.
13.2 Animal Toxicology and/or Pharmacology
Reproductive Toxicology Studies
Reproduction studies have been performed in rats and rabbits at doses up to 3 g/kg/day and 1 g/kg/day, respectively (approximately 50 and 17 times the maximum human dose, based on body weight, mg/kg) and have revealed no evidence of harm to the fetus due to colesevelam hydrochloride.
-
14 CLINICAL STUDIES
14.1 Primary Hyperlipidemia
Colesevelam hydrochloride reduces total cholesterol (TC), LDL-C, apolipoprotein B (Apo B), and non-high-density lipoprotein cholesterol (non-HDL-C) when administered alone or in combination with a statin in patients with primary hyperlipidemia. Approximately 1,600 patients were studied in 9 clinical trials with treatment durations ranging from 4 weeks to 50 weeks. With the exception of one open-label, uncontrolled, long-term extension study, all studies were multicenter, randomized, double-blind, and placebo-controlled. A maximum therapeutic response to colesevelam hydrochloride was achieved within 2 weeks and was maintained during long-term therapy.
Monotherapy
In a study in patients with LDL-C between 130 mg/dL and 220 mg/dL (mean 158 mg/dL), colesevelam hydrochloride was given for 24 weeks in divided doses with the morning and evening meals.
As shown in Table 7, the mean LDL-C reductions were 15% and 18% at the 3.8 g and 4.5 g doses. The respective mean TC reductions were 7% and 10%. The mean Apo B reductions were 12% in both treatment groups. Colesevelam hydrochloride at both doses increased HDL-C by 3%. Increases in TG of 9% to 10% were observed at both colesevelam hydrochloride doses, but the changes were not statistically different from placebo.
Table 7 Response to Colesevelam Hydrochloride Monotherapy in a 24-Week Trial - Percent Change in Lipid Parameters from Baseline Grams/Day N TC LDL-C Apo B HDL-C * Non-HDL-C TG * Placebo 88 +1 0 0 –1 +1 +5 3.8 g (6 tablets) 95 –7 † –15 † –12 † +3 † –10 † +10 4.5 g (7 tablets) 94 –10 † –18 † –12 † +3 –13 † +9 In a study in 98 patients with LDL-C between 145 mg/dL and 250 mg/dL (mean 169 mg/dL), colesevelam hydrochloride 3.8 g was given for 6 weeks as a single dose with breakfast, as a single dose with dinner, or as divided doses with breakfast and dinner. The mean LDL-C reductions were 18%, 15%, and 18% for the 3 dosing regimens, respectively. The reductions with these 3 regimens were not statistically different from one another.
Combination Therapy
Co-administration of colesevelam hydrochloride and a statin (atorvastatin, lovastatin, or simvastatin) in 3 clinical studies demonstrated an additive reduction of LDL-C. The mean baseline LDL-C was 184 mg/dL in the atorvastatin study (range 156 mg/dL to 236 mg/dL), 171 mg/dL in the lovastatin study (range 115 mg/dL to 247 mg/dL), and 188 mg/dL in the simvastatin study (range 148 mg/dL to 352 mg/dL). As demonstrated in Table 8, colesevelam hydrochloride doses of 2.3 g to 3.8 g resulted in an additional 8% to 16% reduction in LDL-C above that seen with the statin alone.
Table 8 Response to Colesevelam Hydrochloride in Combination with Atorvastatin, Simvastatin, or Lovastatin - Percent Change in Lipid Parameters Dose/Day N TC LDL-C Apo B HDL-C * Non-HDL-C TG * Atorvastatin Trial (4-week) Placebo 19 +4 +3 –3 +4 +4 +10 Atorvastatin 10 mg 18 –27 † –38 † –32 † +8 –35 † –24 † Colesevelam hydrochloride 3.8 g/Atorvastatin 10 mg 18 –31 † –48 † –38 † +11 –40 † –1 Atorvastatin 80 mg 20 –39 † –53 † –46 † +6 –50 † –33 † Simvastatin Trial (6-week) Placebo 33 –2 –4 –4 † –3 –2 +6 † Simvastatin 10 mg 35 –19 † –26 † –20 † +3 † –24 † –17 † Colesevelam hydrochloride 3.8 g/ Simvastatin 10 mg 34 –28 † –42 † –33 † +10 † –37 † –12 † Simvastatin 20 mg 39 –23 † –34 † –26 † +7 † –30 † –12 † Colesevelam hydrochloride 2.3 g/ Simvastatin 20 mg 37 –29 † –42 † –32 † +4 † –37 † –12 † Lovastatin Trial (4-week) Placebo 26 +1 0 0 +1 +1 +1 Lovastatin 10 mg 26 –14 † –22 † –16 † +5 –19 † 0 Colesevelam hydrochloride 2.3 g/ Lovastatin 10 mg Together 27 –21 † –34 † –24 † +4 –27 † –1 Colesevelam hydrochloride 2.3 g/ Lovastatin 10 mg Apart 23 –21 † –32 † –24 † +2 –28 † –2 In all 3 studies, the LDL-C reduction achieved with the combination of colesevelam hydrochloride and any given dose of statin therapy was statistically superior to that achieved with colesevelam hydrochloride or that dose of the statin alone. The LDL-C reduction with atorvastatin 80 mg was not statistically significantly different from the combination of colesevelam hydrochloride 3.8 g and atorvastatin 10 mg.
Pediatric Therapy
The safety and efficacy of colesevelam hydrochloride in pediatric patients were evaluated in an 8-week, multi-center, randomized, double-blind, placebo-controlled, parallel-group study followed by an open-label phase, in 194 boys and post-menarchal girls 10 years to 17 years of age (mean age 14.1 years) with HeFH, taking a stable dose of an FDA-approved statin (with LDL-C > 130 mg/dL) or naïve to lipid-lowering therapy (with LDL-C > 160 mg/dL). This study had 3 periods: a single-blind, placebo stabilization period; an 8-week, randomized, double-blind, parallel-group, placebo-controlled treatment period; and an 18-week, open-label treatment period. Forty-seven (24%) patients were taking statins and 147 (76%) patients were statin-naïve at screening. The mean baseline LDL-C at Day 1 was approximately 199 mg/dL.
During the double-blind treatment period, patients were assigned randomly to treatment: Colesevelam hydrochloride 3.8 g/day (n = 64), colesevelam hydrochloride 1.9 g/day (n = 65), or placebo (n = 65). In total, 186 patients completed the double-blind treatment period. After 8 weeks of treatment, colesevelam hydrochloride 3.8 g/day significantly decreased plasma levels of LDL-C, non-HDL-C, TC, and Apo B and significantly increased HDL-C. A moderate, non-statistically significant increase in TG was observed versus placebo (Table 9).
Table 9 Response to Colesevelam Hydrochloride 3.8 g Compared to Placebo in Pediatric Patients 10 Years to 17 Years of Age - Mean Percent Change in Lipid Parameters from Baseline to Week 8 Treatment Difference TC
(N = 128)LDL-C
(N = 128)Apo B
(N = 124)HDL-C
(N = 128)Non-HDL-C
(N = 128)TG *
(N = 128)Colesevelam hydrochloride 3.8 g vs Placebo -7 † -13 † -8 † +6 † -11 † +5 During the open-label treatment period patients were treated with colesevelam hydrochloride 3.8 g/day. In total, 173 (89%) patients completed 26 weeks of treatment. Results at Week 26 were consistent with those at Week 8.
- 16 HOW SUPPLIED/STORAGE AND HANDLING
-
17 PATIENT COUNSELING INFORMATION
Hypertriglyceridemia and Pancreatitis
Inform patients that colesevelam hydrochloride may increase their serum triglycerides which can lead to hypertriglyceridemia and pancreatitis. Instruct patients to discontinue colesevelam hydrochloride and seek prompt medical attention if the symptoms of acute pancreatitis occur (e.g., severe abdominal pain with or without nausea and vomiting) [see Warnings and Precautions (5.1)].
Gastrointestinal
Inform patients that colesevelam hydrochloride may cause bowel obstruction. Instruct patients to promptly discontinue colesevelam hydrochloride and seek medical attention if severe abdominal pain or severe constipation occurs [see Warnings and Precautions (5.2)].
Drug and Vitamin Interactions
Advise patients that colesevelam hydrochloride has drug interactions, and colesevelam hydrochloride may decrease the absorption of fat-soluble vitamins A, D, E, and K. Instruct patients to take oral vitamins at least 4 hours prior to colesevelam hydrochloride. Instruct patients to inform their physician about all the drugs and vitamins that they are prescribed or take over the counter [see Warnings and Precautions (5.3) and Drug Interactions (7)].
Hypertriglyceridemia and Cardiovascular Disease
Inform patients that colesevelam hydrochloride may increase serum triglycerides and that the long-term effect of hypertriglyceridemia on the risk of coronary artery disease is uncertain [see Warnings and Precautions (5.1)].
Administration[see Dosage and Administration ( 2.2, 2.4)]:
Advise patients to take colesevelam hydrochloride tablets with a meal and liquid. Inform patients that colesevelam hydrochloride tablets can be taken as 6 tablets once daily or 3 tablets twice daily.Females of Reproductive Potential
Advise females of reproductive potential that colesevelam hydrochloride may reduce the effectiveness of oral contraceptives, and to take oral contraceptives at least 4 hours before taking colesevelam hydrochloride [see Drug Interactions (7.1) and Use in Specific Populations (8.3)].
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 625 mg, 180 Tablets
-
INGREDIENTS AND APPEARANCE
COLESEVELAM HYDROCHLORIDE
colesevelam hydrochloride tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:69452-158 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength COLESEVELAM HYDROCHLORIDE (UNII: P4SG24WI5Q) (COLESEVELAM - UNII:1XU104G55N) COLESEVELAM HYDROCHLORIDE 625 mg Inactive Ingredients Ingredient Name Strength MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) FERROSOFERRIC OXIDE (UNII: XM0M87F357) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYSORBATE 80 (UNII: 6OZP39ZG8H) TALC (UNII: 7SEV7J4R1U) Product Characteristics Color white Score no score Shape OVAL Size 19mm Flavor Imprint Code COL Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69452-158-25 180 in 1 BOTTLE; Type 0: Not a Combination Product 12/02/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA208670 12/02/2019 Labeler - Bionpharma Inc. (079637826) Registrant - Bionpharma Inc. (079637826) Establishment Name Address ID/FEI Business Operations Patheon Whitby, Ontario Canada 205475333 manufacture(69452-158) , analysis(69452-158) Establishment Name Address ID/FEI Business Operations DPx Fine Chemicals Linz Austria 300398976 api manufacture(69452-158) Establishment Name Address ID/FEI Business Operations OrBion Pharmaceuticals Private Limited 854403569 manufacture(69452-158)


