Label: GENTEAL TEARS (MODERATE)- dextran 70, glycerin, hypromellose solution/ drops
- NDC Code(s): 0065-0426-36, 0065-0426-37
- Packager: Alcon Laboratories, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 15, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- Uses
- Warnings
- Do not use
- When using this product
- STOP USE
- Keep out of reach of children.
- Directions
- Other Information
- Inactive ingredients:
- Questions?
-
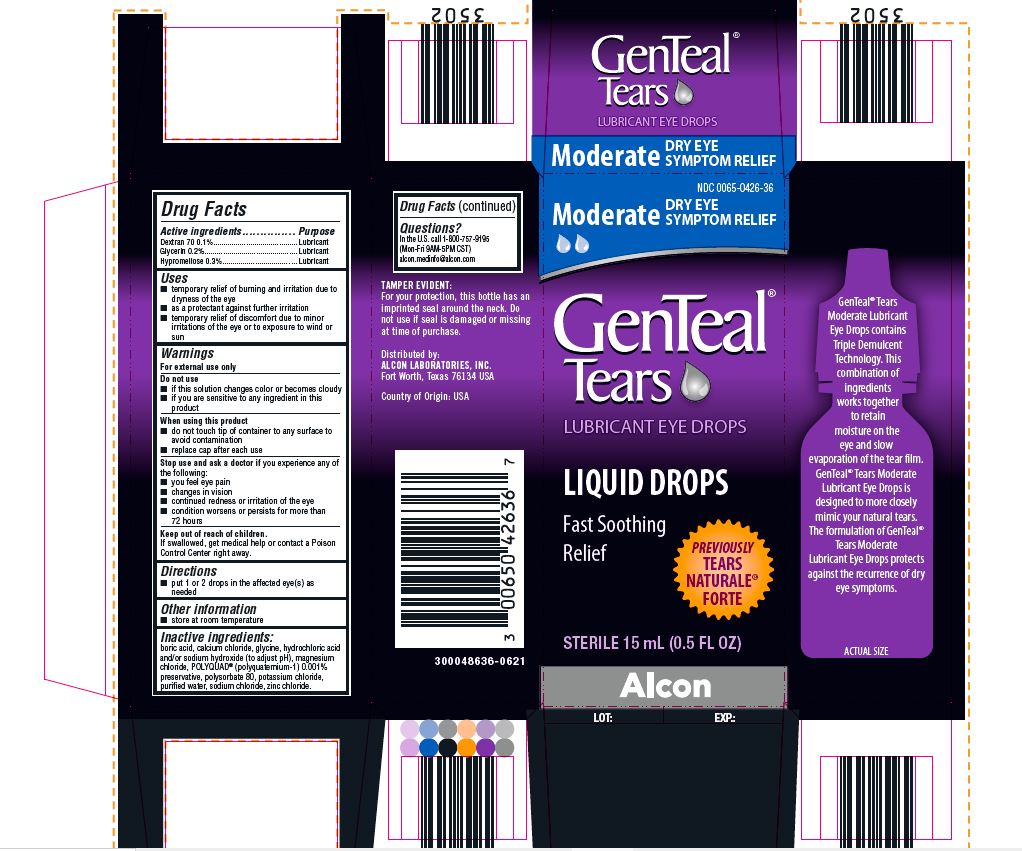
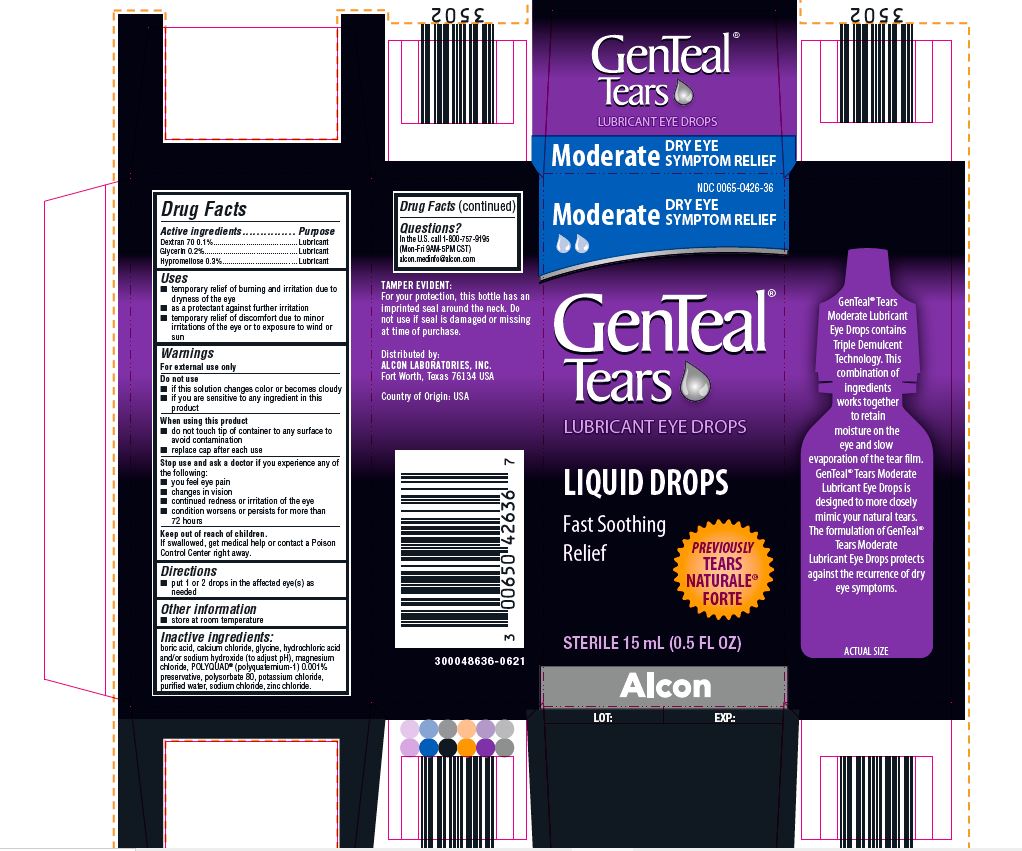
PRINCIPAL DISPLAY PANEL
NDC 0065-0426-36
Moderate DRY EYE SYMPTOM RELIEF
GenTeal® Tears
LUBRICANT EYE DROPS
LIQUID DROPS
Fast Soothing Relief
PREVIOUSLY TEARS NATURALE® FORTE
STERILE 15 mL (0.5 FL OZ)
Alcon
TAMPER EVIDENT:
For your protection, this bottle has an
imprinted seal around the neck. Do
not use if seal is damaged or missing
at time of purchase.
Distributed by:
ALCON LABORATORIES, INC.
Fort Worth, Texas 76134 USA
Country of Origin: USA
300048636-0621
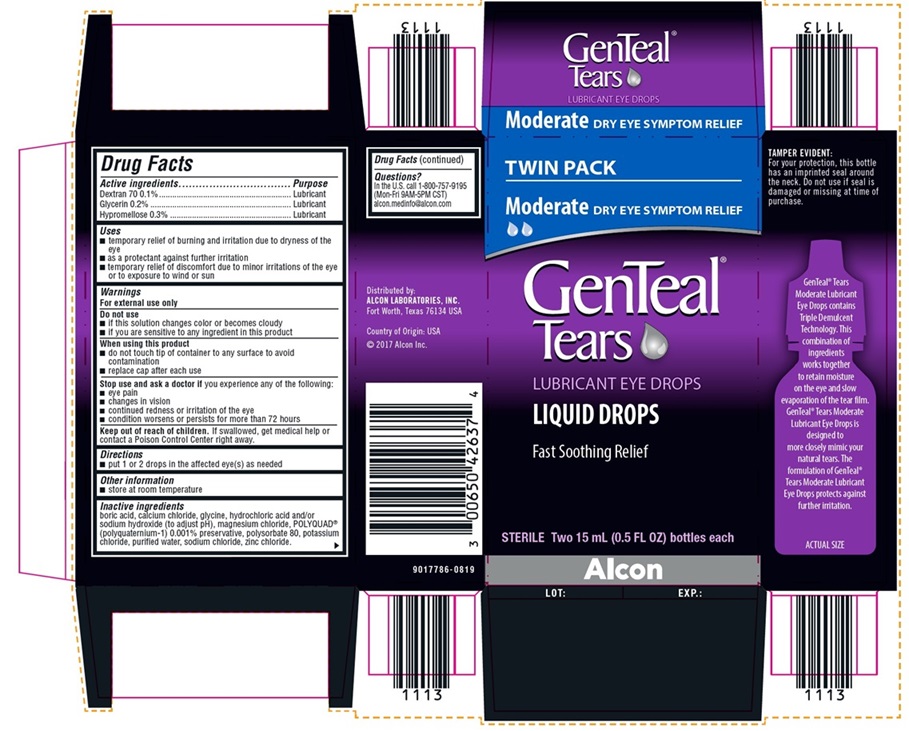
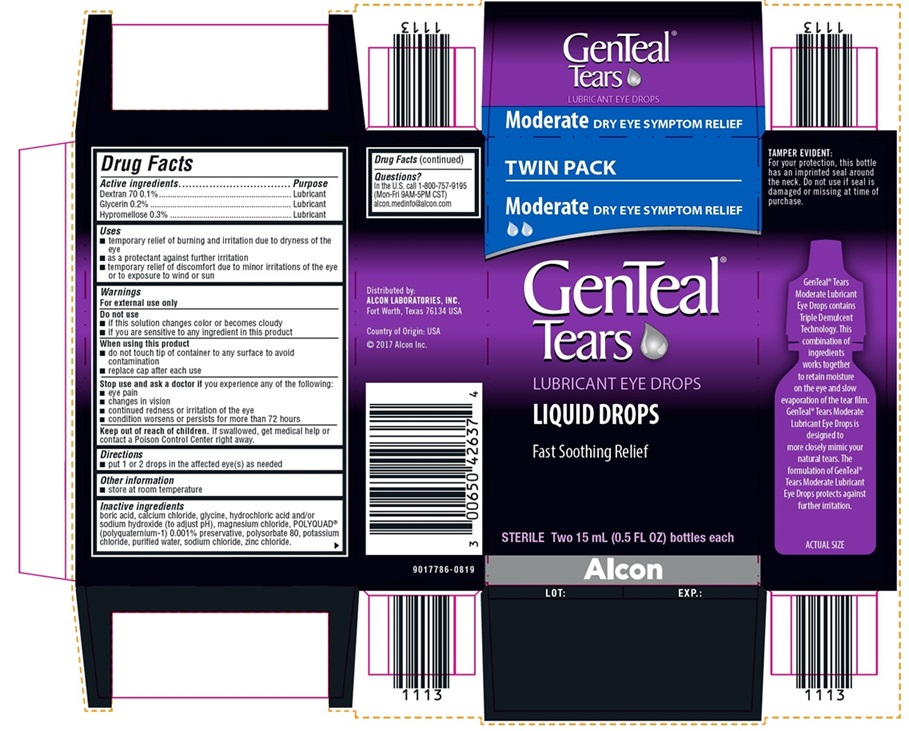
NDC 0065-0426-37
TWIN PACK
Moderate DRY EYE SYMPTOM RELIEF
GenTeal® Tears
LUBRICANT EYE DROPS
LIQUID DROPS
Fast Soothing Relief
STERILE Two 15 mL (0.5 FL OZ) bottles each
Alcon
TAMPER EVIDENT:
For your protection, this bottle has an
imprinted seal around the neck. Do
not use if seal is damaged or missing
at time of purchase.
Distributed by:
ALCON LABORATORIES, INC.
Fort Worth, Texas 76134 USA
Country of Origin: USA
©2017 Alcon Inc.
9017786-0819
NDC 0065-0426-36
Moderate DRY EYE SYMPTOM RELIEF
GenTeal® Tears
LUBRICANT EYE DROPS
LIQUID DROPS
Fast Soothing Relief
STERILE 15 ml (0.5 FL OZ)
STERILE-FOR TOPICAL EYE USE ONLY
Uses: For the temporary relief of burning and irritation due to dryness of the eye and for use as a protectant against further irritation. For the temporary relief of discomfort due to minor irritation of the eye or to exposure to wind or sun. Directions: put 1 or 2 drops in the affected eye(s) as needed.
Warnings: If solution changes color or becomes cloudy, do not use. To avoid contamination, do not touch tip of container to any surface. Replace cap after using. Keep this and all drugs out of the reach of children. See carton for complete Warnings.
INGREDIENTS: Each mL contains: Active: Dextran 70 0.1%, Glycerin 0.2% and Hypromellose 0.3%. Preservative: POLYQUAD* (polyquaternium-1) 0.001%. Inactives: boric acid, calcium chloride, glycine, hydrochloric acid and/or sodium hydroxide (to adjust pH), magnesium chloride, polysorbate 80, potassium chloride, purified water, sodium chloride, zinc chloride.
TAMPER EVIDENT: For your protection, this bottle has an imprinted seal around the neck. Do not use if seal is damaged or missing at time or purchase.
Store at room temperature.
Alcon
Distributed by:
ALCON LABORATORIES, INC.
Fort Worth, Texas 76134 USA
LOT: EXP:
300048635-0621
-
INGREDIENTS AND APPEARANCE
GENTEAL TEARS (MODERATE)
dextran 70, glycerin, hypromellose solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0065-0426 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Dextran 70 (UNII: 7SA290YK68) (Dextran 70 - UNII:7SA290YK68) Dextran 70 1 mg in 1 mL Glycerin (UNII: PDC6A3C0OX) (Glycerin - UNII:PDC6A3C0OX) Glycerin 2 mg in 1 mL Hypromelloses (UNII: 3NXW29V3WO) (Hypromelloses - UNII:3NXW29V3WO) Hypromelloses 3 mg in 1 mL Inactive Ingredients Ingredient Name Strength Boric Acid (UNII: R57ZHV85D4) Calcium Chloride (UNII: M4I0D6VV5M) Glycine (UNII: TE7660XO1C) Hydrochloric Acid (UNII: QTT17582CB) Sodium Hydroxide (UNII: 55X04QC32I) Magnesium Chloride (UNII: 02F3473H9O) Polidronium Chloride (UNII: 6716Z5YR3G) Polysorbate 80 (UNII: 6OZP39ZG8H) Potassium Chloride (UNII: 660YQ98I10) Water (UNII: 059QF0KO0R) Sodium Chloride (UNII: 451W47IQ8X) Zinc Chloride (UNII: 86Q357L16B) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0065-0426-36 1 in 1 CARTON 03/01/2016 1 15 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 2 NDC:0065-0426-37 2 in 1 CARTON 03/01/2016 2 15 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M018 03/01/2016 Labeler - Alcon Laboratories, Inc. (008018525) Establishment Name Address ID/FEI Business Operations Alcon Research LLC 007672236 manufacture(0065-0426)