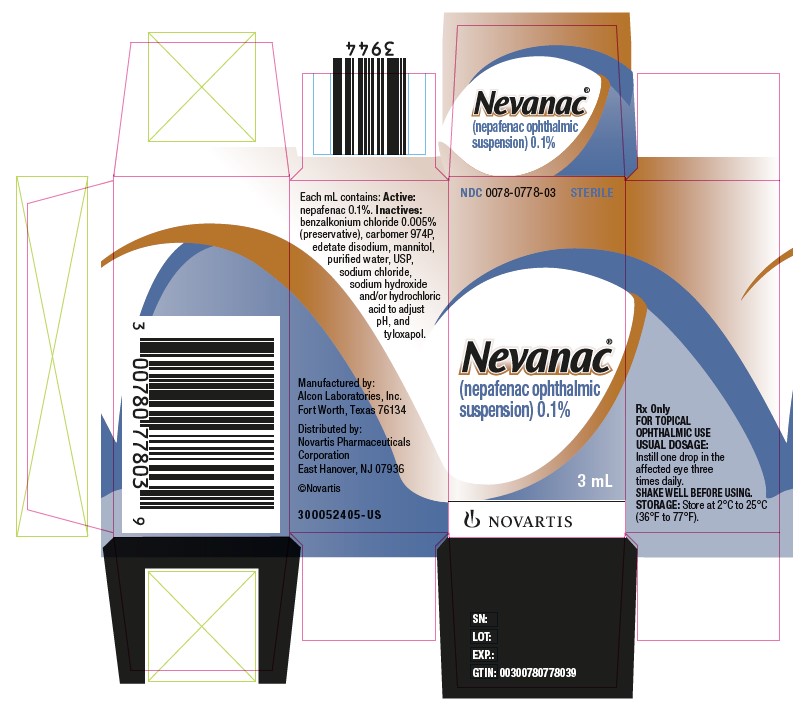
Label: NEVANAC- nepafenac suspension/ drops
- NDC Code(s): 0078-0778-03
- Packager: Novartis Pharmaceuticals Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated April 19, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use NEVANAC safely and effectively. See full prescribing information for NEVANAC.
NEVANAC® (nepafenac ophthalmic suspension) 0.1%, for topical ophthalmic use
Initial U.S. Approval: 2005
INDICATIONS AND USAGE
NEVANAC is a nonsteroidal, anti-inflammatory prodrug indicated for the treatment of pain and inflammation associated with cataract surgery. (1)
DOSAGE AND ADMINISTRATION
One drop of NEVANAC should be applied to the affected eye three times daily beginning 1 day prior to cataract surgery, continued on the day of surgery and through the first 2 weeks of the postoperative period. (2.1)
DOSAGE FORMS AND STRENGTHS
Sterile ophthalmic suspension 0.1%
3 mL in a 4 mL bottle (3)
CONTRAINDICATIONS
Hypersensitivity to any of the ingredients in the formula or to other non-steroidal anti-inflammatory drugs (NSAIDS). (4)
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
Most common adverse reactions (5% to 10%) are capsular opacity, decreased visual acuity, foreign body sensation, increased intraocular pressure (IOP), and sticky sensation. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Novartis Pharmaceuticals Corporation at 1-888-669-6682 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 9/2021
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosing
2.2 Use With Other Topical Ophthalmic Medications
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Increased Bleeding Time
5.2 Delayed Healing
5.3 Corneal Effects
5.4 Contact Lens Wear
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosing
One drop of NEVANAC 0.1% should be applied to the affected eye three times daily beginning 1 day prior to cataract surgery, continued on the day of surgery and through the first 2 weeks of the postoperative period. Shake the container well prior to dosing.
2.2 Use With Other Topical Ophthalmic Medications
NEVANAC 0.1% may be administered in conjunction with other topical ophthalmic medications, such as beta-blockers, carbonic anhydrase inhibitors, alpha-agonists, cycloplegics, and mydriatics.
If more than one topical ophthalmic medication is being used, the medicines must be administered at least 5 minutes apart.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Increased Bleeding Time
With some NSAIDs, including NEVANAC 0.1%, there exists the potential for increased bleeding time due to interference with thrombocyte aggregation. There have been reports that ocularly applied NSAIDs may cause increased bleeding of ocular tissues (including hyphema) in conjunction with ocular surgery.
It is recommended that NEVANAC 0.1% be used with caution in patients with known bleeding tendencies, or who are receiving other medications, which may prolong bleeding time.
5.2 Delayed Healing
Topical NSAIDs, including NEVANAC 0.1%, may slow or delay healing. Topical corticosteroids are also known to slow or delay healing. Concomitant use of topical NSAIDs and topical steroids may increase the potential for healing problems.
5.3 Corneal Effects
Use of topical NSAIDs may result in keratitis. In some susceptible patients, continued use of topical NSAIDs may result in epithelial breakdown, corneal thinning, corneal erosion, corneal ulceration, or corneal perforation. These events may be sight threatening. Patients with evidence of corneal epithelial breakdown should immediately discontinue use of topical NSAIDs, including NEVANAC 0.1%, and should be closely monitored for corneal health.
Postmarketing experience with topical NSAIDs suggests that patients with complicated ocular surgeries, corneal denervation, corneal epithelial defects, diabetes mellitus, ocular surface diseases (e.g., dry eye syndrome), rheumatoid arthritis, or repeat ocular surgeries within a short period of time may be at increased risk for corneal adverse events, which may become sight threatening. Topical NSAIDs should be used with caution in these patients.
Postmarketing experience with topical NSAIDs also suggests that use more than 1 day prior to surgery or use beyond 14 days post-surgery may increase patient risk and severity of corneal adverse events.
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of labeling:
- Increased Bleeding Time [see Warnings and Precautions (5.1)]
- Delayed Healing [see Warnings and Precautions (5.2)]
- Corneal Effects [see Warnings and Precautions (5.3)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to the rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The most frequently reported ocular adverse reactions following cataract surgery were capsular opacity, decreased visual acuity, foreign body sensation, increased intraocular pressure (IOP), and sticky sensation. These reactions occurred in approximately 5% to 10% of patients.
Other ocular adverse reactions occurring at an incidence of approximately 1% to 5% included conjunctival edema, corneal edema, dry eye, lid margin crusting, ocular discomfort, ocular hyperemia, ocular pain, ocular pruritus, photophobia, tearing, and vitreous detachment.
Some of these reactions may be the consequence of the cataract surgical procedure.
Non-ocular adverse reactions reported at an incidence of 1% to 4% included headache, hypertension, nausea/vomiting, and sinusitis.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic Effects
Pregnancy Category C: Reproduction studies performed with nepafenac in rabbits and rats at oral doses up to 10 mg/kg/day have revealed no evidence of teratogenicity due to nepafenac, despite the induction of maternal toxicity. At this dose, the animal plasma exposure to nepafenac and amfenac was approximately 260 and 2400 times human plasma exposure at the recommended human topical ophthalmic dose for rats and 80 and 680 times human plasma exposure for rabbits, respectively. In rats, maternally toxic doses greater than or equal to 10 mg/kg were associated with dystocia, increased post-implantation loss, reduced fetal weights and growth, and reduced fetal survival.
Nepafenac has been shown to cross the placental barrier in rats. There are no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, NEVANAC 0.1% should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Non-Teratogenic Effects
Because of the known effects of prostaglandin biosynthesis inhibiting drugs on the fetal cardiovascular system (closure of the ductus arteriosus), the use of NEVANAC 0.1% during late pregnancy should be avoided.
8.3 Nursing Mothers
Nepafenac is excreted in the milk of lactating rats. It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when NEVANAC 0.1% ophthalmic suspension is administered to a nursing woman.
-
11 DESCRIPTION
NEVANAC® 0.1% is a sterile, topical NSAID prodrug for ophthalmic use. Each mL of NEVANAC 0.1% contains 3 mg of nepafenac. Nepafenac is designated chemically as 2-amino-3-benzoylbenzeneacetamide with an empirical formula of C15H14N2O2. The structural formula of nepafenac is:
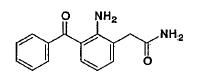
Nepafenac is a yellow crystalline powder. The molecular weight of nepafenac is 254.28 g/mol. NEVANAC, 0.1% is supplied as a sterile, aqueous suspension with a pH approximately of 7.4.
The osmolality of NEVANAC 0.1% is approximately 305 mOsm/kg.
Each mL of NEVANAC 0.1% contains: Active: nepafenac 0.1%. Inactives: benzalkonium chloride 0.005% (preservative), carbomer 974P, edetate disodium, mannitol, purified water, USP, sodium chloride, sodium hydroxide and/or hydrochloric acid to adjust pH, and tyloxapol.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
After topical ocular dosing, nepafenac penetrates the cornea and is converted by ocular tissue hydrolases to amfenac, an NSAID. Amfenac is thought to inhibit the action of prostaglandin H synthase (cyclooxygenase), an enzyme required for prostaglandin production.
12.3 Pharmacokinetics
Low but quantifiable plasma concentrations of nepafenac and amfenac were observed in the majority of subjects 2 and 3 hours post dose, respectively, following bilateral topical ocular 3 times-daily dosing of nepafenac ophthalmic suspension, 0.1%. The mean steady-state Cmax for nepafenac and for amfenac were 0.310 ± 0.104 ng/mL and 0.422 ± 0.121 ng/mL, respectively, following ocular administration.
Nepafenac at concentrations up to 300 ng/mL did not inhibit the in vitro metabolism of 6 specific marker substrates of cytochrome P450 (CYP) isozymes (CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A4). Therefore, drug-drug interactions involving CYP-mediated metabolism of concomitantly administered drugs are unlikely. Drug-drug interactions mediated by protein binding are also unlikely.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Nepafenac has not been evaluated in long-term carcinogenicity studies. Increased chromosomal aberrations were observed in Chinese hamster ovary cells exposed in vitro to nepafenac suspension. Nepafenac was not mutagenic in the Ames assay or in the mouse lymphoma forward mutation assay. Oral doses up to 5,000 mg/kg did not result in an increase in the formation of micronucleated polychromatic erythrocytes in vivo in the mouse micronucleus assay in the bone marrow of mice.
Nepafenac did not impair fertility when administered orally to male and female rats at 3 mg/kg (approximately 90 and 380 times the plasma exposure to the parent drug, nepafenac, and the active metabolite, amfenac, respectively, at the recommended human topical ophthalmic dose).
-
14 CLINICAL STUDIES
In two double-masked, randomized clinical trials in which patients were dosed 3 times daily beginning 1 day prior to cataract surgery, continued on the day of surgery and for the first 2 weeks of the postoperative period, NEVANAC 0.1% demonstrated superior clinical efficacy compared to its vehicle in treating postoperative pain and inflammation.
Patients treated with NEVANAC® 0.1% were less likely to have ocular pain and measurable signs of inflammation (cells and flare) in the early postoperative period through the end of treatment than those treated with its vehicle.
For ocular pain in both studies, a significantly higher percentage of patients (approximately 80%) in the nepafenac group reported no ocular pain on the day following cataract surgery (Day 1) compared to those in the vehicle group (approximately 50%).
Results from clinical studies indicated that NEVANAC 0.1% has no significant effect upon IOP; however, changes in IOP may occur following cataract surgery.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
NEVANAC® 0.1% is supplied in a white, oval, low density polyethylene dispenser with a natural low density polyethylene dispensing plug and gray polypropylene cap. The 1.7 mL fill is presented in an overwrap, which provides tamper evidence to the package. Tamper evidence for the 3 mL fill is provided with a shrink band around the closure and neck area of the package.
3 mL in a 4 mL bottle……………………………………………………………………………NDC 0078-0778-03
Storage: Store at 2°C to 25°C (36°F to 77°F).
-
17 PATIENT COUNSELING INFORMATION
Slow or Delayed Healing
Advise the patient of the possibility that slow or delayed healing may occur while using NSAIDs [see Warnings and Precautions (5.2)].
Avoiding Contamination of the Product
Advise the patient to avoid allowing the tip of the dispensing container to contact the eye or surrounding structures because this could cause the tip to become contaminated by common bacteria known to cause ocular infections. Serious damage to the eye and subsequent loss of vision may result from using contaminated solutions.
Use of the same bottle for both eyes is not recommended with topical eye drops that are used in association with surgery.
Contact Lens Wear
Advise the patient that NEVANAC 0.1% should not be administered while wearing contact lens [see Warnings and Precautions (5.4)].
Intercurrent Ocular Conditions
Advise the patient that if they develop an intercurrent ocular condition (e.g., trauma, or infection) or have ocular surgery, they should immediately seek their physician’s advice concerning the continued use of the multi-dose container [see Warnings and Precautions (5.1)].
Concomitant Topical Ocular Therapy
If more than one topical ophthalmic medication is being used, the medicines must be administered at least 5 minutes apart [see Dosage and Administration (2.2)].
Shake Well Before Use
Advise the patient to shake the container well [see Dosage and Administration (2.1)].
Distributed by:
Novartis Pharmaceuticals Corporation
East Hanover, NJ 07936©Novartis
T2021-99
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NEVANAC
nepafenac suspension/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0078-0778 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NEPAFENAC (UNII: 0J9L7J6V8C) (NEPAFENAC - UNII:0J9L7J6V8C) NEPAFENAC 1 mg in 1 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) MANNITOL (UNII: 3OWL53L36A) CARBOMER HOMOPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: HHT01ZNK31) SODIUM CHLORIDE (UNII: 451W47IQ8X) TYLOXAPOL (UNII: Y27PUL9H56) EDETATE DISODIUM (UNII: 7FLD91C86K) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0078-0778-03 3 mL in 1 BOTTLE; Type 0: Not a Combination Product 07/05/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021862 09/06/2005 Labeler - Novartis Pharmaceuticals Corporation (002147023)