GENTEAL TEARS (MILD)- dextran 70 and hypromellose 2910 solution/ drops
Alcon Laboratories, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Facts
Uses
- temporary relief of burning and irritation due to dryness of the eye
- temporary relief of discomfort due to minor irritations of the eye or to exposure to wind or sun
- as a protectant against further irritation
Do not use
- if solution changes color or becomes cloudy
- if you are sensitive to any ingredient in this product
When using this product
- to avoid contamination, do not touch tip of container to any surface
- replace cap after using
Stop use and ask a doctor if you experience any of the following:
- eye pain
- changes in vision
- continued redness or irritation of the eye
- condition worsens or persists for more than 72 hours
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Inactive ingredients:
POLYQUAD* (polyquaternium-1) 0.001% preservative, potassium chloride, purified water, sodium borate and sodium chloride. May contain hydrochloric acid and/or sodium hydroxide to adjust pH.
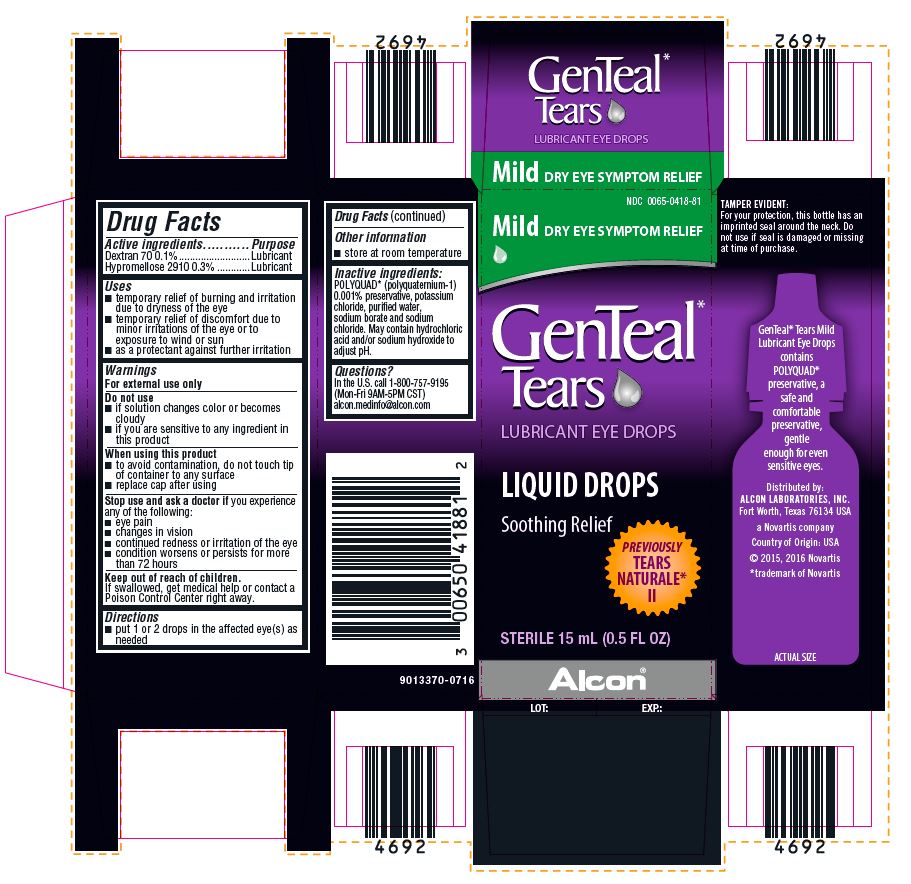
PRINCIPAL DISPLAY PANEL
NDC 0065-0418-81
Mild DRY EYE SYMPTOM RELIEF
GenTeal* Tears
LUBRICANT EYE DROPS
LIQUID DROPS
Soothing Relief
PREVIOUSLY TEARS NATURALE* II
STERILE 15 mL (0.5 FL OZ)
Alcon®
TAMPER EVIDENT:
For your protection, this bottle has an imprinted seal around the neck. Do not use if seal is damaged or missing at time of purchase.
GenTeal* Tears Mild Lubricant Eye Drops contains POLYQUAD* preservative, a safe and comfortable preservative, gentle enough for even sensitive eyes.
Distributed by:
ALCON LABORATORIES, INC.
Fort Worth, Texas 76134 USA
a Novartis company
Country of Origin: USA
© 2015, 2016 Novartis
*trademark of Novartis
ACTUAL SIZE
9013370-0716
LOT:
EXP.:
NDC 0065-0418-81
Mild DRY EYE SYMPTOM RELIEF
GenTeal* Tears
LUBRICANT EYE DROPS
LIQUID DROPS
Soothing Relief
STERILE 15 mL (0.5 FL OZ)
STERILE – FOR TOPICAL EYE USE ONLY
TAMPER EVIDENT: For your protection, this bottle has an imprinted seal around the neck. Do not use if seal is damaged or missing at time of purchase. Store at room temperature.
Alcon®
Distributed by:
ALCON LABORATORIES, INC.
Fort Worth, Texas 76134 USA
© 2015, 2016 Novartis
*trademark of Novartis
LOT/EXP.:
H14443-0716

| GENTEAL TEARS (MILD)
dextran 70 and hypromellose 2910 solution/ drops |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Alcon Laboratories, Inc. (008018525) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Alcon Research LLC | 007672236 | manufacture(0065-0418) | |