Label: ZTLIDO- lidocaine patch
- NDC Code(s): 69557-111-01, 69557-111-03, 69557-111-30
- Packager: Scilex Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated April 21, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ZTLIDO® safely and effectively. See full prescribing information for ZTLIDO®.
ZTLIDO® (lidocaine topical system)
Initial U.S. Approval: 1953RECENT MAJOR CHANGES
Dosage and Administration (2.1) 4/2021 INDICATIONS AND USAGE
ZTLIDO contains lidocaine, an amide local anesthetic, and is indicated for relief of pain associated with post-herpetic neuralgia (PHN) in adults (1).
DOSAGE AND ADMINISTRATION
- Because of the difference in bioavailability of ZTLIDO compared to Lidoderm®, a different dosage strength is required to be administered to the patient. One ZTLIDO (lidocaine topical system) 1.8% provides equivalent lidocaine exposure to one Lidoderm (lidocaine patch 5%). (2.1)
- Apply ZTLIDO to intact skin to cover the most painful area. Apply the prescribed number of topical systems (maximum of 3) only once for up to 12 hours in a 24-hour period. (2.2)
- ZTLIDO may be cut into smaller sizes prior to removal of the release liner. (2.2)
- In patients who are debilitated or have impaired elimination, smaller areas of treatment are recommended. Achieve this by cutting ZTLIDO with scissors into a smaller size prior to removing the release liner. (2.2)
DOSAGE FORMS AND STRENGTHS
ZTLIDO 1.8% is available as a single-dose topical system. (3)
CONTRAINDICATIONS
ZTLIDO is contraindicated in patients with a known history of sensitivity to local anesthetics of the amide type, or to any other component of the product. (4)
WARNINGS AND PRECAUTIONS
- Accidental Exposure: Even a used ZTLIDO topical system contains residual lidocaine after use. It is important for patients to store and dispose of ZTLIDO properly and keep out of the reach of children, pets, and others. (5.1)
- Excessive Dosing/Overexposure: Applying ZTLIDO to larger surface areas or for a longer duration than recommended could lead to increased absorption and high blood concentrations of lidocaine, leading to adverse effects. (5.2)
- Increased Absorption on Non-Intact Skin: May result in higher blood concentrations of lidocaine. (5.2)
- Risk of Overexposure with External Heat Sources: Applying external heat sources to ZTLIDO may result in increased drug exposure. (5.2)
- Methemoglobinemia: Cases of methemoglobinemia have been reported in association with local anesthetic use. (5.3)
- Application Site Reactions: During or immediately after treatment with ZTLIDO, application site reactions may develop. (5.4)
- Hypersensitivity Reactions: Cross sensitivity to ZTLIDO in patients with a history of drug sensitivity to para-aminobenzoic acid (PABA) derivatives is possible. (5.5)
- Eye Exposure: Immediately wash out the eye with water or saline and protect the eye until sensation returns. (5.6).
ADVERSE REACTIONS
Common adverse reactions are application site reactions such as irritation, erythema, and pruritus. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Scilex Pharmaceuticals Inc. at 1-866-SCILEX3 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Class I Antiarrhythmic Drugs: When ZTLIDO is used in patients receiving Class I antiarrhythmic drugs (such as tocainide and mexiletine) the toxic effects are additive and potentially synergistic. Consider risk/benefit before concomitant use. (7.2)
- Local Anesthetic Agents: When ZTLIDO is used concomitantly with other products containing local anesthetic agents, the effects are additive. Consider the amount of drug absorbed from all formulations when local anesthetics are administered concomitantly. (7.3)
USE IN SPECIFIC POPULATIONS
Lactation: Lidocaine is excreted into human milk. Caution should be exercised when ZTLIDO is administered to a nursing mother, especially when administered with other local anesthetics. (8.2)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 4/2021
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Instructions
2.2 Treatment of Post-Herpetic Neuralgia
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Accidental Exposure
5.2 Excessive Dosing/Overexposure to Lidocaine
5.3 Methemoglobinemia
5.4 Application Site Reactions
5.5 Hypersensitivity Reactions
5.6 Eye Exposure
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
7.1 Drugs That May Cause Methemoglobinemia When Used with ZTLIDO
7.2 Antiarrhythmic Drugs
7.3 Local Anesthetics
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Instructions
Because of the difference in bioavailability of ZTLIDO compared to Lidoderm (lidocaine patch 5%), a different dosage strength is required to be administered to the patient. One ZTLIDO (lidocaine topical system) 1.8% provides equivalent lidocaine exposure to one Lidoderm (lidocaine patch 5%) [see Clinical Pharmacology (12.3)].
When ZTLIDO is used concomitantly with other products containing local anesthetic agents, the total amount of drug absorbed from all formulations must be considered.
Clearly instruct and advise patients:
- to wash hands immediately after handling ZTLIDO and to avoid contact with eyes [see Warnings and Precautions (5.6)].
- to store ZTLIDO inside the sealed envelope out of the reach of children, pets, and others and to apply immediately after removal from the envelope.
- to fold used ZTLIDO so that the adhesive side sticks to itself and to safely discard used ZTLIDO or pieces of cut ZTLIDO where children and pets cannot get to them [see Warnings and Precautions (5.1)].
- to never apply external heat sources, such as heating pads or electric blankets, directly to ZTLIDO, because plasma lidocaine levels are increased. ZTLIDO can be applied, however, to the administration site after moderate heat exposure, such as 15 minutes of heating pad exposure on a medium setting [see Warnings and Precautions (5.2), Clinical Pharmacology (12.3)].
- that clothing may be worn over the area of application.
- that ZTLIDO may be used during moderate exercise, such as biking for 30 minutes.
- that ZTLIDO may be exposed to water, such as showering for 10 minutes or immersion for 15 minutes.
- to dry the topical system, after water exposure, by gently patting the skin, not by rubbing the skin or topical system.
- that ZTLIDO topical systems that have lifted at the edges may be reattached by pressing firmly on the edges and that fully detached topical systems may be reapplied as originally directed.
- that if a ZTLIDO topical system comes off completely and will not stick to patient's skin, to remove and properly dispose of the used ZTLIDO and to apply a new ZTLIDO topical system for a total duration of 12 hours of used and new topical system together.
- that if irritation or a burning sensation occurs during application, to remove ZTLIDO and to not reapply until the irritation subsides [see Warnings and Precautions (5.4)].
2.2 Treatment of Post-Herpetic Neuralgia
Advise patients to apply ZTLIDO to intact skin to cover the most painful area and to apply the prescribed number of topical systems (maximum of 3), only once for up to 12 hours within a 24-hour period (12 hours on and 12 hours off) [see Warnings and Precautions (5.2)].
In patients who are debilitated or have impaired elimination, smaller areas of treatment are recommended. Achieve this by cutting ZTLIDO with scissors into a smaller size prior to removing the release liner.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Accidental Exposure
A used ZTLIDO topical system contains residual lidocaine after use. The potential exists for a small child or a pet to suffer serious adverse effects from chewing or ingesting a new or used ZTLIDO. It is important for patients to store and dispose of ZTLIDO properly, and keep out of the reach of children, pets, and others [see Dosage and Administration (2.1)].
5.2 Excessive Dosing/Overexposure to Lidocaine
Lidocaine toxicity can be expected at lidocaine blood concentrations above 5 mcg/mL. The blood concentration of lidocaine is determined by the rate and extent of lidocaine absorption and elimination. Longer duration of application, application of more than the recommended number of ZTLIDO, smaller patients, or impaired elimination may all contribute to increasing the blood concentration of lidocaine.
If lidocaine overdose is suspected, check drug blood concentration. Management of overdose includes close monitoring, supportive care, and symptomatic treatment [see Overdosage (10)].
Improper Application and Duration of Use: Application of more than the recommended number of ZTLIDO or applying ZTLIDO for longer than the recommended wearing time (12 hours of every 24 hours) could result in increased absorption and high blood concentrations of lidocaine, leading to adverse reactions. Advise patients on proper application and duration.
Hepatic Disease: Impaired elimination may contribute to increasing blood concentrations of lidocaine. Patients with severe hepatic disease are at greater risk of developing toxic blood concentrations of lidocaine because of their inability to metabolize lidocaine normally.
Use on Non-Intact Skin: Application to broken or inflamed skin, although not tested, may result in higher blood concentrations of lidocaine from increased absorption. ZTLIDO is only recommended for use on intact skin. Advise patients not to apply ZTLIDO to non-intact skin.
External Heat Sources: External heat sources may increase drug exposure, leading to overexposure to lidocaine. Advise patients not to apply external heat sources to ZTLIDO during administration [see Clinical Pharmacology (12.3)].
5.3 Methemoglobinemia
Cases of methemoglobinemia have been reported in association with local anesthetic use. Although all patients are at risk for methemoglobinemia, patients with glucose-6-phosphate dehydrogenase deficiency, congenital or idiopathic methemoglobinemia, cardiac or pulmonary compromise, infants under 6 months of age, and concurrent exposure to oxidizing agents or their metabolites are more susceptible to developing clinical manifestations of the condition. If local anesthetics must be used in these patients, close monitoring for symptoms and signs of methemoglobinemia is recommended.
Signs of methemoglobinemia may occur immediately or may be delayed some hours after exposure, and are characterized by a cyanotic skin discoloration and/or abnormal coloration of the blood. Methemoglobin levels may continue to rise; therefore, immediate treatment is required to avert more serious central nervous system and cardiovascular adverse effects, including seizures, coma, arrhythmias, and death. Discontinue ZTLIDO and any other oxidizing agents. Depending on the severity of the signs and symptoms, patients may respond to supportive care, i.e., oxygen therapy, hydration. A more severe clinical presentation may require treatment with methylene blue, exchange transfusion, or hyperbaric oxygen.
5.4 Application Site Reactions
During or immediately after treatment with ZTLIDO, the skin at the site of application may develop blisters, bruising, burning sensation, depigmentation, dermatitis, discoloration, edema, erythema, exfoliation, irritation, papules, petechia, pruritus, vesicles, or may be the locus of abnormal sensation. These reactions are generally mild and transient, resolving spontaneously within a few minutes to hours. If application site reactions occur while the topical system is being worn, advise the patient to remove ZTLIDO and not to reapply until skin reactions subside.
5.5 Hypersensitivity Reactions
Patients allergic to para-aminobenzoic acid (PABA) derivatives (procaine, tetracaine, benzocaine, etc.) have not shown cross-sensitivity to lidocaine. However, be aware of the potential for cross-sensitivity in patients allergic to PABA derivatives, especially if the etiologic agent is uncertain. Manage hypersensitivity reactions by conventional means. The detection of sensitivity by skin testing is of doubtful value.
5.6 Eye Exposure
The contact of ZTLIDO with eyes, although not studied, should be avoided based on findings of severe eye irritation with the application of similar products in animals. If eye contact occurs, immediately wash out the eye with water or saline and protect the eye (such as, eye glasses/eye wear) until sensation returns.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling:
- Excessive Dosing/Overexposure to Lidocaine [see Warnings and Precautions (5.2)]
- Methemoglobinemia [see Warnings and Precautions (5.3)]
- Application Site Reactions [see Warnings and Precautions (5.4)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.5)]
- Eye Irritation [see Warnings and Precautions (5.6)]
The following adverse reactions from voluntary reports or clinical studies have been reported with lidocaine. Because some of these reactions were reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Skin and subcutaneous tissues: blisters, bruising, burning sensation, depigmentation, dermatitis, discoloration, edema, erosions, erythema, exfoliation, flushing, irritation, papules, petechia, pruritus, vesicles, and abnormal sensation.
Immune system: angioedema, bronchospasm, dermatitis, dyspnea, hypersensitivity, laryngospasm, pruritus, shock, and urticaria.
Central Nervous System: lightheadedness, nervousness, apprehension, euphoria, confusion, dizziness, drowsiness, tinnitus, blurred or double vision, sensations of heat, cold or numbness, twitching, tremors, convulsions, unconsciousness, somnolence, respiratory depression and arrest.
Cardiovascular: bradycardia, hypotension, and cardiovascular collapse leading to arrest.
Other: asthenia, disorientation, headache, hyperesthesia, hypoesthesia, metallic taste, nausea, pain exacerbated, paresthesia, taste alteration, and vomiting.
-
7 DRUG INTERACTIONS
7.1 Drugs That May Cause Methemoglobinemia When Used with ZTLIDO
Patients who are administered local anesthetics may be at increased risk of developing methemoglobinemia when concurrently exposed to the following drugs, which could include other local anesthetics:
Examples of Drugs Associated with Methemoglobinemia:
Class Examples Nitrates/Nitrites nitric oxide, nitroglycerin, nitroprusside, nitrous oxide Local anesthetics articaine, benzocaine, bupivacaine, lidocaine, mepivacaine, prilocaine, procaine, ropivacaine, tetracaine Antineoplastic agents cyclophosphamide, flutamide, hydroxyurea, ifosfamide, rasburicase Antibiotics dapsone, nitrofurantoin, para-aminosalicyclic acid, sulfonamides Antimalarials chloroquine, primaquine Anticonvulsants phenobarbital, phenytoin, sodium valproate Other drugs acetaminophen, metoclopramide, quinine, sulfasalazine -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
The limited human data with lidocaine in pregnant woman are not sufficient to inform drug-associated risk for major birth defects and miscarriage.
The use of lidocaine for labor neuraxial analgesia has not been associated with an increased incidence of adverse fetal effects either during delivery or during the neonatal period (see Data). Should ZTLIDO be used concomitantly with other products containing lidocaine, consider total drug doses contributed by all formulations.
In a published animal reproduction study, pregnant rats administered lidocaine by continuous subcutaneous infusion at a dose approximately 45 times the maximum recommended daily dose (MRDD) of 108 mg in ZTLIDO during the period of organogenesis resulted in lower fetal body weights. In a published animal reproduction study, pregnant rats administered lidocaine, containing 1:100,000 epinephrine, injected into the masseter muscle of the jaw or into the gum of the lower jaw at 0.5 times the MRDD on Gestation Day 11 resulted in developmental delays in neonates (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies carry some risk of birth defects, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Human Data
In 22 parturient women given 1.5% lidocaine epidural anesthesia, there were no effects on neonatal behavior, using the early neonatal neurobehavioral scale (ENNS). Neuraxial analgesia also did not affect fetal heart rate, beat-to-beat variability, or uterine activity.
Animal Data
Reproductive studies with lidocaine have been performed in rats at doses up to 30 mg/kg (2.7 times the maximum recommended daily dose [MRDD] of 108 mg from ZTLIDO on a mg/m2 basis) subcutaneously and have revealed no evidence of harm to the fetus due to lidocaine.
In a published study, lidocaine administered to pregnant rats by continuous subcutaneous infusion during the period of organogenesis at 100, 250, and 500 mg/kg/day, did not produce any structural abnormalities, but did result in lower fetal weights at 500 mg/kg/day dose (approximately 45 times the MRDD on a mg/m2 basis) in the absence of maternal toxicity.
In a published study, lidocaine containing 1:100,000 epinephrine at a dose of 6 mg/kg (approximately 0.5 times the MRDD on a mg/m2 basis) injected into the masseter muscle of the jaw or into the gum of the lower jaw of pregnant Long-Evans hooded rats on Gestation Day 11 resulted in developmental delays in the neonates. Developmental delays were observed for negative geotaxis, static righting reflex, visual discrimination response, sensitivity and response to thermal and electrical shock stimuli, and water maze acquisition. The developmental delays of the neonatal animals were transient, with responses becoming comparable to untreated animals later in life. The clinical relevance of these animal data is uncertain.
8.2 Lactation
Risk Summary
Lidocaine is excreted into human milk. When lidocaine was used as an epidural anesthetic for cesarean section in 27 women, a milk:plasma ratio of 1.07 was observed using AUC values. Lactating women undergoing a dental procedure had a 0.4 milk:plasma ratio. In another dental procedure study, a single patient was administered 20 mg of lidocaine and the milk:plasma ratio was reported as 1.1 at five to six hours after injection. These data, and the low concentrations of lidocaine in the plasma after topical administration of ZTLIDO in recommended doses, suggest that a small amount of lidocaine would be ingested orally by a suckling infant. However, caution should be exercised when ZTLIDO is administered to a nursing mother, especially when administered with other local anesthetics.
8.5 Geriatric Use
Clinical studies of ZTLIDO did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be done with caution, usually starting at the low end of the dosing range (e.g., a single topical system), reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
-
10 OVERDOSAGE
Lidocaine overdose from cutaneous absorption is rare, but could occur. If there is any suspicion of lidocaine overdose, check drug blood concentration. The management of overdose includes close monitoring, supportive care, and symptomatic treatment. Dialysis is of negligible value in the treatment of acute overdose with lidocaine.
In the absence of massive topical overdose or oral ingestion, evaluation of symptoms of toxicity should include consideration of other etiologies for the clinical effects, or overdosage from other sources of lidocaine or other local anesthetics.
-
11 DESCRIPTION
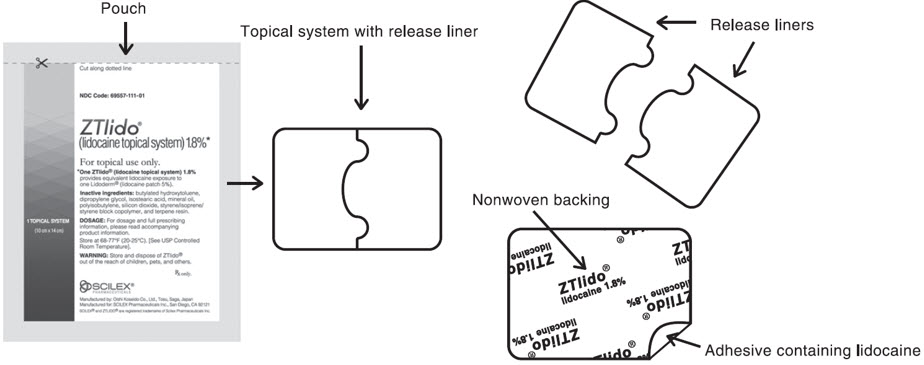
ZTLIDO (lidocaine topical system) 1.8% is a single-layer, drug-in-adhesive topical delivery system comprised of an adhesive material containing 36 mg lidocaine, which is applied to a pliable nonwoven cloth backing and covered with a polyethylene terephthalate film release liner. The release liner is removed prior to application to the skin. The size of ZTLIDO is 10 cm × 14 cm × 0.08 cm.
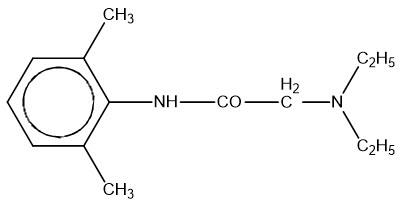
Lidocaine, an amide local anesthetic, is chemically designated as acetamide, 2-(diethylamino)-N-(2,6-dimethylphenyl), has an octanol:water partition ratio of 43 at pH 7.4, and has the following structure:
Each ZTLIDO contains 36 mg of lidocaine (18 mg per gram adhesive) in a non-aqueous base and also contains the following inactive ingredients: butylated hydroxytoluene, dipropylene glycol, isostearic acid, mineral oil, polyisobutylene, silicone dioxide, styrene/isoprene/styrene block copolymer, and terpene resin.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Lidocaine is an amide local anesthetic. Lidocaine blocks sodium ion channels required for the initiation and conduction of neuronal impulses.
12.2 Pharmacodynamics
The penetration of lidocaine into intact skin after application of ZTLIDO is sufficient to produce an analgesic effect, but less than the amount necessary to produce a complete sensory block.
12.3 Pharmacokinetics
ZTLIDO has different bioavailability compared to Lidoderm. In a single-dose, crossover study conducted in 53 healthy volunteers, ZTLIDO (lidocaine topical system) 1.8% demonstrated equivalent exposure (AUC) and peak concentration (Cmax) of lidocaine to Lidoderm (lidocaine patch 5%).
Absorption
The amount of lidocaine systemically absorbed from ZTLIDO is directly related to both the duration of application and the surface area over which it is applied. In a pharmacokinetic study, three ZTLIDO topical systems were applied over an area of 420 cm2 of intact skin on the backs of normal healthy volunteers for 12 hours. Blood samples were drawn for determination of lidocaine concentration during the topical system application and for 12 hours after removal of topical systems. The results are summarized in Table 1.
Table 1 Mean ± SD Absorption of lidocaine from ZTLIDO Healthy Volunteers (n = 54, 12-hour application time) Topical System Application Site Area (cm2) Cmax (ng/mL) Tmax (hr)* - *
- median (min, max)
3 Topical systems of ZTLIDO
(108 mg)Back 420 75.1 ± 28.0 13.9 (4.0, 18.0) Repeated application of three Lidoderm patches simultaneously for 12 hours (recommended maximum daily dose), once per day for three days, indicated that the lidocaine concentration does not increase with daily use. The mean plasma pharmacokinetic profile for the 15 healthy volunteers is shown in Figure 1.
Figure 1
Mean lidocaine blood concentrations after three consecutive daily applications of three Lidoderm patches simultaneously for 12 hours per day in healthy volunteers (n = 15).
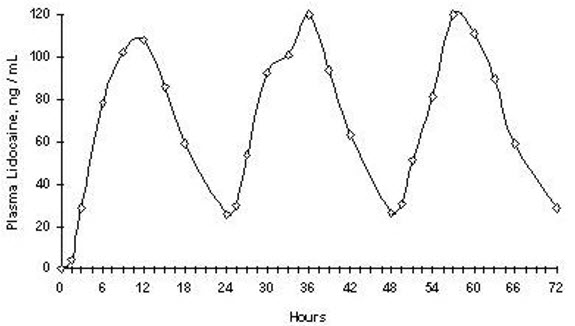
The pharmacokinetics of ZTLIDO (n = 3 topical systems) was assessed in 12 healthy volunteers with exposure to external heat source (heating pad at medium setting applied for 20 minutes at Time 0 and 8.5 hours) or undergoing moderate exercise (cycling for 30 minutes at a heart rate of 108 bpm at Time 0, 2.5, 5.5 and 8.5 hours) and compared to pharmacokinetics of ZTLIDO at rest. Exposure to external heat at 0 and 8.5 hours results in increased peak plasma levels of lidocaine with a mean (SD) of 160.3 ± 100.1 ng/mL versus the peak plasma levels observed at rest with a mean (SD) of 97.6 ± 36.9 ng/mL. For this reason, instruct patients not to apply heating pads directly to ZTLIDO. Concentrations returned to normal within 4 hours after the heat was removed. No clinically relevant differences in systemic absorption were observed under exercise conditions with a mean (SD) peak plasma concentration of 90.5 ± 25.4 ng/mL.
A separate study in 12 healthy volunteers showed that there was no effect on ZTLIDO pharmacokinetics when the topical system is applied to the administration site after external heat exposure (heating pad at medium setting applied for 15 minutes prior to the topical system application) or after engagement in exercise (walking at a moderate pace on a treadmill for approximately 20 minutes beginning approximately 30 minutes prior to the topical system application).
Distribution
When lidocaine is administered intravenously to healthy volunteers, the volume of distribution is 0.7 to 2.7 L/kg (mean 1.5 ± 0.6 SD, n = 15). At concentrations produced by application of ZTLIDO, lidocaine is approximately 70% bound to plasma proteins, primarily alpha-1-acid glycoprotein. At much higher plasma concentrations (1 to 4 µg/mL of free base), the plasma protein binding of lidocaine is concentration dependent. Lidocaine crosses the placental and blood brain barriers, presumably by passive diffusion.
Elimination
Metabolism:
It is not known if lidocaine is metabolized in the skin. Lidocaine is metabolized rapidly by the liver to a number of metabolites, including monoethylglycinexylidide (MEGX) and glycinexylidide (GX), both of which have pharmacologic activity similar to, but less potent than that of lidocaine. A minor metabolite, 2,6-xylidine, has unknown pharmacologic activity. The blood concentration of this metabolite is negligible following application of ZTLIDO. Following intravenous administration, MEGX and GX concentrations in serum range from 11 to 36% and from 5 to 11% of lidocaine concentrations, respectively.
Excretion:
Lidocaine and its metabolites are excreted by the kidneys. Less than 10% of lidocaine is excreted unchanged. The half-life of lidocaine elimination from the plasma following IV administration is 81 to 149 minutes (mean 107 ± 22 SD, n = 15). The systemic clearance is 0.33 to 0.90 L/minute (mean 0.64 ± 0.18 SD, n = 15).
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Long-term studies in animals specifically designed to evaluate the carcinogenic potential of lidocaine or ZTLIDO have not been conducted.
A metabolite, 2,6-xylidine, has been found to be carcinogenic in rats. The clinical significance is not known.
Mutagenesis
Lidocaine HCl was not mutagenic in the in vitro bacterial reverse mutagenicity assay (Ames test). Lidocaine HCl was not clastogenic in the in vitro chromosome aberration assay with human lymphocytes or in the in vivo mouse micronucleus test.
Impairment of Fertility
In a published study, female Sprague-Dawley rats were treated subcutaneously with lidocaine via osmotic pumps starting two weeks prior to mating, and reproductive effects were assessed. Rats dosed up to the high dose of 500 mg/kg/day (approximately 45 times the MRDD on a mg/m2 basis) showed no effects on copulatory rate, pregnancy rate, or the numbers of corpora lutea or implantations.
-
14 CLINICAL STUDIES
Single-dose treatment with lidocaine patch (currently preferred dosage form term for a patch is topical system) was compared to treatment with vehicle patch (without lidocaine), and to no treatment (observation only) in a double-blind, crossover clinical trial with 35 post-herpetic neuralgia patients. Pain intensity and pain relief scores were evaluated periodically for 12 hours. Lidocaine patch performed statistically better than vehicle patch in terms of pain intensity from 4 to 12 hours.
Multiple-dose, two-week treatment with lidocaine patch was compared to vehicle patch (without lidocaine) in a double-blind, crossover clinical trial of withdrawal-type design conducted in 32 patients, who were considered as responders to the open-label use of lidocaine patch prior to the study. The constant type of pain was evaluated but not the pain induced by sensory stimuli (dysesthesia). Statistically significant differences favoring lidocaine patch were observed in terms of time to exit from the trial (14 versus 3.8 days at p-value <0.001), daily average pain relief, and patient's preference of treatment. About half of the patients also took oral medication commonly used in the treatment of post-herpetic neuralgia. The extent of use of concomitant medication was similar in the two treatment groups.
Based on a clinical study in 54 subjects with ZTLIDO, 47 subjects (87%) had adhesion scores of 0 (≥ 90% adhered) for all evaluations performed every 3 hours during the 12 hours of administration, 7 subjects (13%) had adhesion scores of 1 (≥ 75% to < 90% adhered) for at least one evaluation, and no subjects had scores of 2 or greater (< 75% adhered).
-
16 HOW SUPPLIED/STORAGE AND HANDLING
ZTLIDO (lidocaine topical system) 1.8% is available as the following:
Carton of 30 topical systems, packaged into individual child-resistant envelopes.
NDC 69557-111-30
Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Wash hands thoroughly immediately after handling the topical system. Upon removal, fold the topical system in half so that the adhesive side sticks to itself. Discard used ZTLIDO and pieces of cut ZTLIDO in a manner that prevents accidental contact or ingestion by children, pets or others [see Dosage and Administration (2.2), Warnings and Precautions (5.1)].
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Accidental Exposure and Disposal
Advise patients to store ZTLIDO out of the reach of children, pets, and others. Advise patients to dispose of used ZTLIDO by folding used ZTLIDO so that the adhesive side sticks to itself and safely discarding used ZTLIDO or pieces of cut ZTLIDO where children, pets, and others cannot come in contact with them [see Warnings and Precautions (5.1)].
Proper Application
Advise patients:
- not to apply more than the prescribed number (up to 3 ZTLIDO) [see Dosage and Administration (2.2), Warnings and Precautions (5.2)].
- not to wear ZTLIDO longer than the recommended wearing time (12 hours of every 24 hours) [see Dosage and Administration (2.2), Warnings and Precautions (5.2)].
- to apply ZTLIDO to intact skin [see Warnings and Precautions (5.2)].
- to reattach by pressing firmly on the edges of ZTLIDO that are lifting. If a ZTLIDO topical system comes off completely and will not stick to patient's skin, it should be removed and properly disposed of and a new ZTLIDO topical system should be applied for a total duration of 12 hours of used and new topical system together [see Dosage and Administration (2.1, 2.2)].
MethemoglobinemiaInform patients that use of local anesthetics may cause methemoglobinemia, a serious condition that must be treated promptly. Advise patients or caregivers to stop use and seek immediate medical attention if they or someone in their care experience the following signs or symptoms: pale, gray, or blue colored skin (cyanosis); headache; rapid heart rate; shortness of breath; lightheadedness; or fatigue [see Warnings and Precautions (5.3)].
Application Site ReactionsInform patients that skin irritation and other skin reactions may occur at the site of ZTLido application. If skin reactions occur during wear, instruct patients to remove ZTLido and not to reapply until the skin reaction subsides [see Warnings and Precautions (5.4)]
Eye ExposureAdvise patients to wash hands immediately after handling ZTLIDO and to avoid contact with eyes. Instruct patients to, if eye contact should occur, immediately wash out the eye with water or saline and protect the eye until sensation returns [see Dosage and Administration (2.1), Warnings and Precautions (5.6)].
-
SPL UNCLASSIFIED SECTION
Manufactured for:
Scilex Pharmaceuticals Inc.
Palo Alto, CA 94303
USAZTLIDO® is a trademark owned by Scilex Pharmaceuticals Inc.
Patented. See: www.scilexpharma.com/patents
© 2021 Scilex Pharmaceuticals Inc. All rights reserved.
SCILEX®
PHARMACEUTICALSSCILEX® and ZTLIDO® are registered trademarks of Scilex Pharmaceuticals Inc.
-
PATIENT PACKAGE INSERT
This Patient Information has been approved by the U.S. Food and Drug Administration. Issued: 4/2021 PATIENT INFORMATION
ZTLIDO® (ZEE-TEE-LIE-DOH)
(lidocaine topical system)What is ZTLIDO? ZTLIDO is a prescription medicine used for relief of pain from damaged nerves (neuropathic pain) that follows healing of shingles. It is not known if ZTLIDO is safe and effective in children. Do not use ZTLIDO if you: - have a history of allergic reactions to numbing medicines (anesthetics). Ask your healthcare provider if you are not sure.
- are allergic to any of the ingredients in ZTLIDO. See the end of this leaflet for a complete list of ingredients in ZTLIDO.
Before using ZTLIDO, tell your healthcare provider about all your medical conditions, including if you: - have liver problems
- have heart or lung problems
- are allergic to para-aminobenzoic acid (PABA) medicines such as procaine, tetracaine, or benzocaine
- have been told that you have glucose-6-phosphate dehydrogenase deficiency
- were born with (congenital) methemoglobinemia or have had methemoglobinemia from an unknown cause
- are pregnant or plan to become pregnant. It is not known if ZTLIDO will harm your unborn baby.
- are breastfeeding or plan to breastfeed. ZTLIDO can pass into your breast milk. Talk to your healthcare provider about the best way to feed your baby if you use ZTLIDO.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Especially, tell your healthcare provider if you are using other lidocaine containing products or anesthetic medicines. How should I use ZTLIDO? Read the Instructions for Use at the end of this Patient Information leaflet for information about how to apply the ZTLIDO topical system. - Use ZTLIDO exactly as your healthcare provider tells you to use it.
- Do not apply more than your prescribed number of ZTLIDO. You may apply up to 3 ZTLIDO topical systems at one time.
- A ZTLIDO may be worn only 1 time for up to 12 hours within a 24-hour period (12 hours on and 12 hours off).
- Apply ZTLIDO to intact skin only. Do not apply ZTLIDO to skin that is not intact, such as skin that is cut, scraped, burned, or irritated.
- ZTLIDO topical systems that come off completely or are lifting at the edges may be reattached by firmly pressing down on the topical system or on the lifted areas.
- If ZTLIDO lifts off the skin or detaches more than 1 time, do not try to reattach it. Throw it away as described below.
- If the ZTLIDO you are wearing comes off completely, and will not stick to your skin, throw it away as described below.
In either of these cases, you may apply a replacement (a new) ZTLIDO topical system. Take off the replacement ZTLIDO at your usual removal time. The total time you may wear the used and replacement ZTLIDO is 12 hours.
- You may wear clothing over the ZTLIDO application site.
- Do not apply external heat sources, such as heating pads or electric blankets, directly on ZTLIDO. This may cause increased levels of lidocaine in your blood. You may apply ZTLIDO to a treatment site after moderate heat exposure, such as after 15 minutes of heating pad use on a medium setting.
- ZTLIDO may be used during moderate exercise, such as biking for 30 minutes.
- ZTLIDO may be worn in water such as showering for 10 minutes or bathing for 15 minutes.
- After ZTLIDO is exposed to water, pat the topical system dry. Do not rub the topical system.
- After using ZTLIDO, fold the used ZTLIDO so that the sticky sides stick together. Safely throw away used ZTLIDO and any pieces of cut ZTLIDO where children and pets cannot get to them.
- Wash your hands right away after handling ZTLIDO (after you apply ZTLIDO, when you try to re-attach it, or when you remove it.)
- If you start feeling irritation or burning when applying ZTLIDO, remove the ZTLIDO. Do not reapply ZTLIDO until the irritation or burning goes away.
- If you apply more than 3 ZTLIDO topical systems or apply ZTLIDO for longer than 12 hours of a 24-hour period, call your healthcare provider.
What should I avoid while using ZTLIDO? - Avoid contact of your hands and fingers with your eyes while handling ZTLIDO. See "Contact of ZTLIDO with your eyes" below.
What are the possible side effects of ZTLIDO? ZTLIDO may cause serious side effects, including: -
Lidocaine overdose can happen if you apply more than the prescribed number of ZTLIDO, applying ZTLIDO for longer than 12 hours, have liver problems, use ZTLIDO on skin that is not intact, or if you apply external heat sources directly on ZTLIDO. This can result in increased levels of lidocaine in your blood.
- Do not apply more than the prescribed number of ZTLIDO.
- Do not wear ZTLIDO longer than 12 hours.
- Do not apply ZTLIDO on skin that is not intact, such as skin that is cut, scraped, burned, or irritated.
- Do not apply external heat sources directly to ZTLIDO. See "How should I use ZTLIDO?" for more information about how to properly use external heat sources when using ZTLIDO.
-
A serious blood problem called methemoglobinemia. Methemoglobinemia is a serious blood problem where to much methemoglobin is produced in the blood. Methemoglobinemia can happen with the use of local anesthetics and may not let enough oxygen reach the organs and tissues in your body. Anyone who uses or receives local anesthetics is at risk for methemoglobinemia, but certain people are more likely to have serious medical problems and need to be closely monitored by their healthcare provider during treatment with ZTLIDO, including:
- people with glucose-6-phosphate dehydrogenase deficiency
- people with heart of lung problems
- babies under 6 months of age
- people who were born with (congenital) or who have had methemoglobinemia from an unknown cause
- people exposed to certain chemicals at the same time that they use or receive a local anesthetic
You or your caregiver should stop using ZTLIDO and get medical help right away if you develop any of the following signs or symptoms:
- pale, gray, or blue colored skin
- headache
- rapid heart beat
- shortness of breath
- lightheadedness
- tiredness
- Application site reactions. Skin irritation and other skin reactions at the ZTLIDO application site are common and are usually mild. These reactions can happen during or right after treatment with ZTLIDO. Application site reactions will usually go away within a few minutes to hours. Symptoms of application site reactions may include:
- blisters
- bruising
- burning or abnormal sensation
- change or loss of color of your skin
- swelling, redness, and pain of the skin
- peeling or flaking of skin
- irritation
- pimple-like raised skin
- itching
If you develop a skin reaction while wearing ZTLIDO, remove it. Do not reapply ZTLIDO until the site reaction goes away. - Allergic reactions can happen if you have a history of allergic reactions to numbing medicines (anesthetics). Tell your healthcare provider right away if you have any symptoms of an allergic reaction such as swelling or shortness of breath.
- Contact of ZTLIDO with your eyes can happen if you touch your eyes while handling the topical system and can cause severe eye irritation. Avoid eye contact with your hands and fingers while handling ZTLIDO. Wash your hands right away after handling ZTLIDO. If the medicine in ZTLIDO comes in contact with your eye, wash out your eye with water or saline right away. Protect the eye (for example eye glasses or eye wear) until the numbness goes away.
These are not all the possible side effects of ZTLIDO. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. How should I store ZTLIDO? - Store ZTLIDO at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep ZTLIDO topical system in the original packaging unit until ready for use.
- Each ZTLIDO topical system comes in a child-resistant envelope.
Keep ZTLIDO and all medicines out of the reach of children, pets, and others. General information about the safe and effective use of ZTLIDO. Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use ZTLIDO for a condition for which it was not prescribed. Do not give ZTLIDO to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about ZTLIDO that is written for health professionals. What are the ingredients in ZTLIDO? Active ingredient: lidocaine Inactive ingredients: butylated hydroxytoluene, dipropylene glycol, isostearic acid, mineral oil, polyisobutylene, silicone dioxide, styrene/isoprene/styrene block copolymer, and terpene resin. Manufactured for: Scilex Pharmaceuticals Inc., Palo Alto, CA 94303 ZTLIDO® is a trademark owned by Scilex Pharmaceuticals Inc. © 2021 Scilex Pharmaceuticals Inc. All rights reserved. SCILEX®
PHARMACEUTICALSFor more information call [1-844-SCILEX1] or visit www.ZTLIDO.com. -
INSTRUCTIONS FOR USE
Instructions for Use
ZTLIDO® (ZEE-TEE-LIE-DOH)
(lidocaine topical system)Read this Instructions for Use before you start using ZTLIDO and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical treatment or condition.
Important information:
- One ZTLIDO (lidocaine topical system) 1.8% provides equivalent lidocaine exposure to one Lidoderm® (lidocaine patch 5%).
- ZTLIDO is for use on intact skin only.
- ZTLIDO comes in a child-resistant envelope. Do not open the ZTLIDO envelope until you are ready to use it.
- Apply the prescribed number of ZTLIDO topical systems at one time. Clothing may be worn over ZTLIDO.
- You should only use a maximum of 3 ZTLIDO topical systems at a time.
- Keep your prescribed number of ZTLIDO topical systems on for up to 12 hours within a 24-hour period (12 hours on and 12 hours off).
- ZTLIDO may be used during moderate exercise, such as biking for 30 minutes.
- ZTLIDO may be worn in water such as showering for 10 minutes or bathing for 15 minutes.
- After ZTLIDO is exposed to water, pat the topical system dry. Do not rub the topical system.
- ZTLIDO topical systems that have come off completely or are lifting at the edges may be re-attached to the affected area by firmly pressing down on the topical system or on the lifted areas.
- If ZTLIDO lifts off the skin or detaches more than 1 time, do not try to re-attach it. Throw it away (See Step 6).
- If the ZTLIDO you are wearing comes off completely, and will not stick to your skin, throw it away (see Step 6).
Wash your hands well right away after handling ZTLIDO (when you apply ZTLIDO, when you re-attach it, or when you remove it). Avoid eye contact with your hands and fingers while handling ZTLIDO.
Figure A: ZTLIDO Topical System
Applying your ZTLIDO topical system:
Step 1: Select the application site. - ZTLIDO should only be applied to clean, dry, and intact skin to cover the most painful area.
Step 2: Using scissors, carefully cut the envelope along the dotted line and open it to remove ZTLIDO. - Do not use ZTLIDO if it is damaged. Throw it away and get a new one.
- ZTLIDO may be cut into smaller sizes with scissors prior to removal of the transparent release liner.
Step 3a: Remove the transparent release liner before applying ZTLIDO to the skin. Apply ZTLIDO right away after removing the transparent release liner. Apply ZTLIDO only to intact skin. 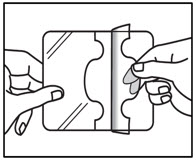
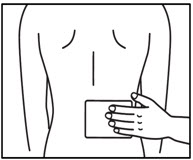
Step 3b: Place the adhesive side of ZTLIDO to skin, while not touching the sticky side. Smooth the ZTLIDO using your hands and firmly press to make sure it sticks well to skin. Step 4: Wash your hands well right away after applying ZTLIDO. - Avoid contact of your hands or fingers with your eyes until after you wash your hands.
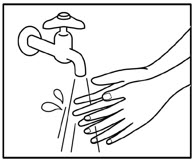
Removing your ZTLIDO topical system:
How to reattach ZTLIDO or apply a replacement ZTLIDO:
- If the ZTLIDO you are wearing comes off completely or lifts at the edges, reattach to the affected area by pressing firmly on the edges of the topical system or lifted areas. If ZTLIDO lifts off the skin or comes off completely more than 1 time, do not try to re-attach it. Throw away the used ZTLIDO as instructed above in Step 6.
- If the ZTLIDO you are wearing comes off completely and will not stick to your skin, throw away the used ZTLIDO as instructed above in Step 6.
- Pat dry the area of skin if ZTLIDO came off while swimming or showering.
- Apply a replacement ZTLIDO the same way you would apply a new ZTLIDO as described above in Steps 1 through 6.
- Remove the replacement ZTLIDO at your usual removal time as described above in Steps 5 and 6.
- The total time you may wear the used and replacement ZTLIDO is 12 hours.
Manufactured for:
Scilex Pharmaceuticals Inc.
Palo Alto, CA 94303
USAZTLIDO® is a trademark owned by Scilex Pharmaceuticals Inc.
© 2021 Scilex Pharmaceuticals Inc. All rights reserved.SCILEX®
PHARMACEUTICALSRevised: 4/2021
-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - lidocaine patch 1.8% CARTON LABEL
NDC 69557-111-30
ZTlido™
(lidocaine topical system) 1.8%*
For topical use only.
*One ZTlido™ (lidocaine topical system) 1.8% provides equivalent lidocaine exposure to one Lidoderm® (lidocaine patch 5%).
Inactive ingredients: butylated hydroxytoluene, dipropylene glycol, isostearic acid, mineral oil, polyisobutylene, silicone dioxide, styrene/isoprene/styrene block copolymer, and terpene resin.
DOSAGE: For dosage and full prescribing information, please read accompanying product information.
Store at 68-77°F (20-25°C). [See USP Controlled Room Temperature].
WARNING: Store and dispose of ZTlido™ out of the reach of children, pets, and others.
30 TOPICAL SYSTEMS
30 Envelopes Containing 1 Topical System Each
SCILEX ™ Rx Only
PHARMACEUTICALS
Manufactured by: Oishi Koseido Co., Ltd., Tosu, Saga, Japan
Manufactured for: SCILEX Pharmaceuticals Inc., San Diego, CA 92121
Active Ingredient Made in Spain
-
INGREDIENTS AND APPEARANCE
ZTLIDO
lidocaine patchProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:69557-111 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 36 mg Inactive Ingredients Ingredient Name Strength DIPROPYLENE GLYCOL (UNII: E107L85C40) MINERAL OIL (UNII: T5L8T28FGP) ISOSTEARIC ACID (UNII: X33R8U0062) POLYISOBUTYLENE (1100000 MW) (UNII: FLT10CH37X) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) POLYISOBUTYLENE (55000 MW) (UNII: TQ77WR8A02) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69557-111-30 30 in 1 CARTON 04/26/2018 1 NDC:69557-111-01 1 in 1 POUCH; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 2 NDC:69557-111-03 3 in 1 CARTON 04/26/2018 2 1 in 1 POUCH; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA207962 04/26/2018 Labeler - Scilex Pharmaceuticals Inc. (078286225) Establishment Name Address ID/FEI Business Operations Oishi Koseido Co., Ltd. 710700410 manufacture(69557-111) , pack(69557-111) , label(69557-111) , analysis(69557-111)