INFLUENZA PLUS- eucalyptus globulus, ipecacuanha, aconitum napellus, influenzinum, respiratory syncytial virus nosode (rsv), belladonna, bryonia (alba), eupatorium perfoliatum, gelsemium sempervirens, kreosotum, veratrum viride liquid
Deseret Biologicals, Inc.
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
DRUG FACTS:
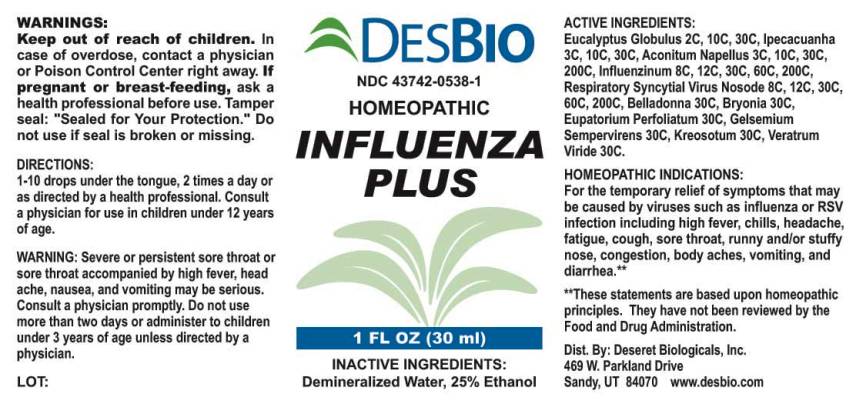
ACTIVE INGREDIENTS:
Eucalyptus Globulus 2C, 10C, 30C, Ipecacuanha 3C, 10C, 30C, Aconitum Napellus 3C, 10C, 30C, 200C, Influenzinum 8C, 12C, 30C, 60C, 200C, Respiratory Syncytial Virus Nosode (RSV) 8C, 12C, 30C, 60C, 200C, Belladonna 30C, Bryonia (Alba) 30C, Eupatorium Perfoliatum 30C, Gelsemium Sempervirens 30C, Kreosotum 30C, Veratrum Viride 30C.
HOMEOPATHIC INDICATIONS:
For the temporary relief of symptoms that may be caused by viruses such as influenza or RSV infection including high fever, chills, headache, fatigue, cough, sore throat, runny and/or stuffy nose, congestion, body aches, vomiting, and diarrhea.**
**These statements are based upon traditional homeopathic practice. They have not been reviewed by the Food and Drug Administration.
WARNINGS:
Keep out of reach of children. In case of overdose, contact a physician or Poison Control Center right away.
If pregnant or breast-feeding, seek advice of a health professional before use.
Tamper seal: "Sealed for Your Protection." Do not use if seal is broken or missing.
Warning: Severe or persistent sore throat accompanied by high fever, headache, nausea, and vomiting may be serious. Consult a physician promptly. Do not use more than two days or administer to children under 3 years of age unless directed by a physician.
KEEP OUT OF REACH OF CHILDREN:
Keep out of reach of children. In case of overdose, contact a physician or Poison Control Center right away.
DIRECTIONS:
1-10 drops under the tongue, 2 times a day or as directed by a health professional. Consult a physician for use in children under 12 years of age.
HOMEOPATHIC INDICATIONS:
For the temporary relief of symptoms that may be caused by viruses such as influenza or RSV infection including high fever, chills, headache, fatigue, cough, sore throat, runny and/or stuffy nose, congestion, body aches, vomiting, and diarrhea.**
**These statements are based upon traditional homeopathic practice. They have not been reviewed by the Food and Drug Administration.
| INFLUENZA PLUS
eucalyptus globulus, ipecacuanha, aconitum napellus, influenzinum, respiratory syncytial virus nosode (rsv), belladonna, bryonia (alba), eupatorium perfoliatum, gelsemium sempervirens, kreosotum, veratrum viride liquid |
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
| Labeler - Deseret Biologicals, Inc. (940741853) |
| Registrant - Apotheca Company (844330915) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Apotheca Company | 844330915 | manufacture(43742-0538) , api manufacture(43742-0538) , label(43742-0538) , pack(43742-0538) | |