DOK- docusate sodium capsule
Major Pharmaceuticals
----------
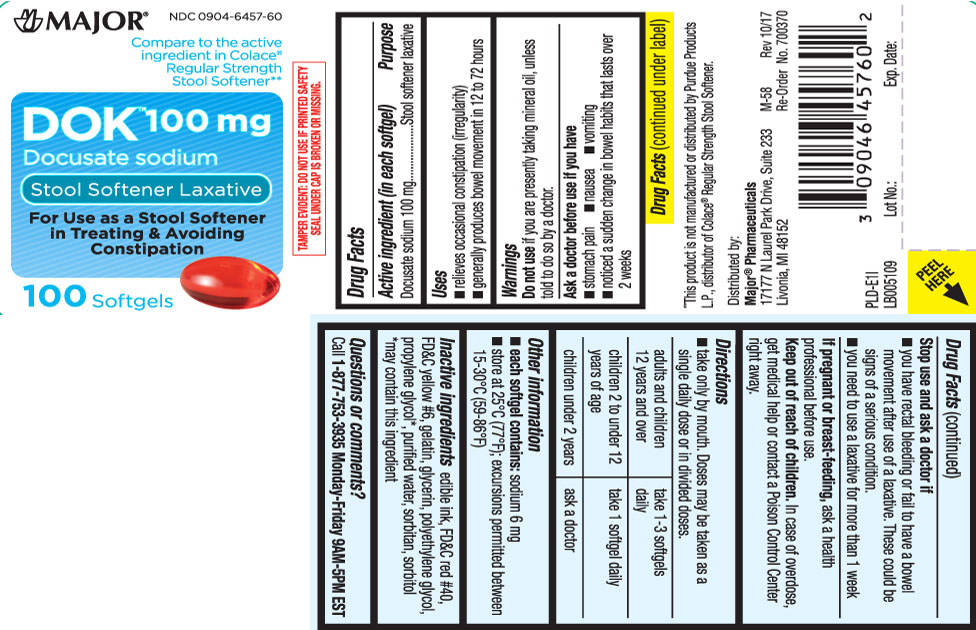
Drug Facts
Uses
- relieves occasional constipation (irregularity)
- generally produces bowel movement in 12 to 72 hours
Warnings
Ask a doctor before use if you have
- stomach pain
- nausea
- vomiting
- noticed a sudden change in bowel habits that last over 2 weeks
Directions
- take only by mouth. Doses may be taken as a single daily dose or in divided doses.
| adults and children 12 years and over | take 1 to 3 softgels daily. |
| children 2 to under 12 years of age | take 1 softgel daily |
| children under 2 years | ask a doctor |
Other information
- each softgel contains: sodium 6 mg
- store at 25ºC (77ºF);excursions permitted between 15-30ºC (59-86ºF)
Inactive ingredients
edible ink, FD&C Red #40, FD&C Yellow #6, gelatin, glycerin, polyethylene glycol, propylene glycol*, purified water sorbitan, sorbitol
*contains one or more of these ingredients
Principal Display Panel
Compare to the active ingredient in Colace® Regular Strength Stool Softener**
DOK™ 100 mg
Docusate sodium
Stool Softener Laxative
For Use as a Stool Softener in Treating & Avoiding Constipation
Softgels
**This product is not manufactured or distributed bt Purdue Products L.P., distributor of Colace® Regular Strength Stool Softener.
TAMPER EVIDENT: DO NOT USE IF PRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING.
Distributed by:
Major® Pharmaceuticals
17177 N Laurel Park Drive, Suite 233
Livonia, MI 48152
| DOK
docusate sodium capsule |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Major Pharmaceuticals (191427277) |
Revised: 11/2023
Document Id: 41db8501-ddfc-4c28-93d5-e9919ade3f12
Set id: a1392a23-c431-4851-ba5d-ee72ca886f06
Version: 12
Effective Time: 20231127
Major Pharmaceuticals