GEMCITABINE- gemcitabine hydrochloride injection, powder, lyophilized, for solution
Athenex Pharmaceutical Division, LLC.
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use GEMCITABINE FOR INJECTION safely and effectively. See full prescribing information for GEMCITABINE FOR INJECTION.
GEMCITABINE for injection, for intravenous use Initial U.S. Approval: 1996 RECENT MAJOR CHANGES
INDICATIONS AND USAGEGemcitabine for Injection is a nucleoside metabolic inhibitor indicated:
DOSAGE AND ADMINISTRATIONGemcitabine for Injection is for intravenous use only.
DOSAGE FORMS AND STRENGTHSGemcitabine for Injection, USP is a white to off-white lyophilized powder available in sterile single-dose vials containing 200 mg or 1 gram gemcitabine. (
3)
CONTRAINDICATIONSPatients with a known hypersensitivity to gemcitabine. (
4)
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONSThe most common adverse reactions for the single agent (≥20%) are nausea/vomiting, anemia, increased aspartate aminotransferase (AST), increased alanine aminotransferase (ALT), neutropenia, increased alkaline phosphatase, proteinuria, fever, hematuria, rash, thrombocytopenia, dyspnea, and edema. (
6.1)
See 17 for PATIENT COUNSELING INFORMATION. Revised: 2/2020 |
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
1.1 Ovarian Cancer
Gemcitabine for Injection in combination with carboplatin is indicated for the treatment of patients with advanced ovarian cancer that has relapsed at least 6 months after completion of platinum-based therapy.
1.2 Breast Cancer
Gemcitabine for Injection in combination with paclitaxel is indicated for the first-line treatment of patients with metastatic breast cancer after failure of prior anthracycline-containing adjuvant chemotherapy, unless anthracyclines were clinically contraindicated.
1.3 Non-Small Cell Lung Cancer
Gemcitabine for Injection in combination with cisplatin is indicated for the first-line treatment of patients with inoperable, locally advanced (Stage IIIA or IIIB) or metastatic (Stage IV) non-small cell lung cancer (NSCLC).
1.4 Pancreatic Cancer
Gemcitabine for Injection is indicated as first-line treatment for patients with locally advanced (nonresectable Stage II or Stage III) or metastatic (Stage IV) adenocarcinoma of the pancreas. Gemcitabine for Injection is indicated for patients previously treated with fluorouracil.
2 DOSAGE AND ADMINISTRATION
2.1 Ovarian Cancer
Recommended Dose and Schedule
The recommended dosage of Gemcitabine for Injection is 1000 mg/m 2intravenously over 30 minutes on Days 1 and 8 of each 21-day cycle in combination with carboplatin AUC 4 administered intravenously on Day 1 after Gemcitabine for Injection administration. Refer to carboplatin prescribing information for additional information.
Dosage Modifications
Recommended Gemcitabine for Injection dosage modifications for myelosuppression are described in Tables 1and 2 [see Warnings and Precautions ( 5.2)] . Refer to the recommended dosage modifications for non-hematologic adverse reactions [see Dosage and Administration ( 2.5)].
| Treatment Day | Absolute Neutrophil Count
(x 106/L) | Platelet Count
(x 106/L) | Dosage Modification | |
| Day 1 | Greater than or equal to 1500 | And | Greater than or equal to 100,000 | None |
| Less than 1500 | Or | Less than 100,000 | Delay Treatment Cycle | |
| Day 8 | Greater than or equal to 1500 | And | Greater than or equal to 100,000 | None |
| 1000 to 1499 | Or | 75,000 to 99,999 | 50% of full dose | |
| Less than 1000 | Or | Less than 75,000 | Hold |
| Occurrence | Myelosuppression During Treatment Cycle | Dosage Modification |
| Initial Occurrence |
| Permanently reduce Gemcitabine for Injection to 800 mg/m 2on Days 1 and 8 |
| Subsequent Occurrence | If any of the above toxicities occur after the initial dose reduction: | Permanently reduce Gemcitabine for Injection to 800 mg/m 2on Day 1 only |
2.2 Breast Cancer
Recommended Dose and Schedule
The recommended dosage of Gemcitabine for Injection is 1250 mg/m 2intravenously over 30 minutes on Days 1 and 8 of each 21-day cycle in combination with paclitaxel 175 mg/m 2administered as a 3-hour intravenous infusion on Day 1 before Gemcitabine for Injection administration. Refer to paclitaxel prescribing information for additional information.
Dosage Modifications
Recommended Gemcitabine for Injection dosage modifications for myelosuppression are described in Table 3 [see Warnings and Precautions ( 5.2)] . Refer to the recommended dosage modifications for non-hematologic adverse reactions [see Dosage and Administration ( 2.5)].
| Treatment Day | Absolute Neutrophil Count
(x 106/L) | Platelet Count
(x 106/L) | Dosage Modification | |
| Day 1 | Greater than or equal to 1500 | And | Greater than or equal to 100,000 | None |
| Less than 1500 | Or | Less than 100,000 | Hold | |
| Day 8 | Greater than or equal to 1200 | And | Greater than 75,000 | None |
| 1000 to 1199 | Or | 50,000 to 75,000 | 75% of full dose | |
| 700 to 999 | And | Greater than or equal to 50,000 | 50% of full dose | |
| Less than 700 | Or | Less than 50,000 | Hold |
2.3 Non-Small Cell Lung Cancer
Recommended Dose and Schedule
28-day schedule
The recommended dosage of Gemcitabine for Injection is 1000 mg/m 2intravenously over 30 minutes on Days 1, 8, and 15 of each 28-day cycle in combination with cisplatin 100 mg/m 2administered intravenously on Day 1 after Gemcitabine for Injection administration.
21-day schedule
The recommended dosage of Gemcitabine for Injection is 1250 mg/m 2intravenously over 30 minutes on Days 1 and 8 of each 21-day cycle in combination with cisplatin 100 mg/m 2administered intravenously on Day 1 after Gemcitabine for Injection administration.
Refer to cisplatin prescribing information for additional information.
2.4 Pancreatic Cancer
Recommended Dose and Schedule
The recommended dosage of Gemcitabine for Injection is 1000 mg/m 2intravenously over 30 minutes. The recommended treatment schedule is as follows:
- Weeks 1 to 8: weekly dosing for the first 7 weeks followed by one week rest.
- After week 8: weekly dosing on Days 1, 8, and 15 of each 28-day cycle.
Dosage Modifications
Recommended dosage modifications for Gemcitabine for Injection for myelosuppression are described in Table 4[see Warnings and Precautions ( 5.2)] . Refer to the recommended dosage modifications for non-hematologic adverse reactions [see Dosage and Administration ( 2.5)].
| Absolute Neutrophil Count
(x 106/L) | Platelet Count
(x 106/L) | Dosage Modification | |
| Greater than or equal to 1000 | And | Greater than or equal to 100,000 | None |
| 500 to 999 | Or | 50,000 to 99,999 | 75% of full dose |
| Less than 500 | Or | Less than 50,000 | Hold |
2.5 Dosage Modifications for Non-Hematologic Adverse Reactions
Permanently discontinue Gemcitabine for Injection for any of the following:
- Unexplained dyspnea or evidence of severe pulmonary toxicity [see Warnings and Precautions ( 5.3)]
- Hemolytic Uremic Syndrome (HUS) or severe renal impairment [see Warnings and Precautions ( 5.4)]
- Severe hepatic toxicity [see Warnings and Precautions ( 5.5)]
- Capillary leak syndrome (CLS) [see Warnings and Precautions ( 5.8)]
- Posterior reversible encephalopathy syndrome (PRES) [see Warnings and Precautions ( 5.9)]
Withhold Gemcitabine for Injection or reduce dose by 50% for other Grade 3 or 4 non-hematological adverse reactions until resolved. No dose modifications are recommended for alopecia, nausea, or vomiting.
2.6 Preparation
- Gemcitabine for Injection vials contain no antimicrobial preservatives and are intended for single use only.
- Gemcitabine for Injection is a cytotoxic drug. Follow applicable special handling and disposal procedures. 1
- Exercise caution and wear gloves when preparing Gemcitabine for Injection solutions. Immediately wash the skin thoroughly or rinse the mucosa with copious amounts of water if Gemcitabine for Injection contacts the skin or mucus membranes. Death has occurred in animal studies due to dermal absorption.
- Reconstitute the 200 mg vial with 5 mL and the 1 gram vial with 25 mL of 0.9% Sodium Chloride Injection, USP to yield a Gemcitabine for Injection concentration of 38 mg/mL. Reconstituted Gemcitabine for Injection is a clear, colorless to light straw-colored solution.
- Visually inspect reconstituted product for particulate matter and discoloration. Discard if particulate matter or discoloration is observed.
- Withdraw the calculated dose from the vial and discard any unused portion.
- Prior to administration, dilute the reconstituted solution with 0.9% Sodium Chloride Injection, USP to a minimum final concentration of at least 0.1 mg/mL.
- Store Gemcitabine for Injection solutions (reconstituted and diluted) at controlled room temperature of 20°C to 25°C (68°F to 77°F). Do not refrigerate as crystallization can occur. Discard Gemcitabine for Injection solutions if not used within 24 hours after reconstitution.
- No incompatibilities have been observed with infusion bottles or polyvinyl chloride bags and administration sets.
3 DOSAGE FORMS AND STRENGTHS
Gemcitabine for Injection, USP is a white to off-white lyophilized powder available in sterile single-dose vials containing 200 mg or 1 gram gemcitabine.
4 CONTRAINDICATIONS
Gemcitabine for Injection is contraindicated in patients with a known hypersensitivity to gemcitabine. Reactions include anaphylaxis [see Adverse Reactions ( 6.1)].
5 WARNINGS AND PRECAUTIONS
5.1 Schedule-Dependent Toxicity
In clinical trials evaluating the maximum tolerated dose of Gemcitabine for Injection, prolongation of the infusion time beyond 60 minutes or more frequent than weekly dosing resulted in an increased incidence of clinically significant hypotension, severe flu-like symptoms, myelosuppression, and asthenia. The half-life of Gemcitabine for Injection is influenced by the length of the infusion [see Clinical Pharmacology ( 12.3)] . Refer to the recommended Gemcitabine for Injection dosage [see Dosage and Administration ( 2.1, 2.2, 2.3, 2.4)] .
5.2 Myelosuppression
Myelosuppression manifested by neutropenia, thrombocytopenia, and anemia occurs with Gemcitabine for Injection as a single agent and the risks are increased when Gemcitabine for Injection is combined with other cytotoxic drugs. In clinical trials, Grade 3-4 neutropenia, anemia, and thrombocytopenia occurred in 25%, 8%, and 5%, respectively of the 979 patients who received single agent Gemcitabine for Injection. The frequencies of Grade 3-4 neutropenia, anemia, and thrombocytopenia varied from 48% to 71%, 8% to 28%, and 5% to 55%, respectively, in patients receiving Gemcitabine for Injection in combination with another drug [see Adverse Reactions ( 6.1)].
Prior to each dose of Gemcitabine for Injection, obtain a complete blood count (CBC) with a differential and a platelet count. Modify the dosage as recommended [see Dosage and Administration ( 2.1, 2.2, 2.3, 2.4)] .
5.3 Pulmonary Toxicity and Respiratory Failure
Pulmonary toxicity, including interstitial pneumonitis, pulmonary fibrosis, pulmonary edema, and adult respiratory distress syndrome (ARDS), has been reported. In some cases, these pulmonary events can lead to fatal respiratory failure despite the discontinuation of therapy. The onset of pulmonary symptoms may occur up to 2 weeks after the last dose of Gemcitabine for Injection [see Adverse Reactions ( 6.1, 6.2)] .
Permanently discontinue Gemcitabine for Injection in patients who develop unexplained dyspnea, with or without bronchospasm, or evidence of severe pulmonary toxicity.
5.4 Hemolytic Uremic Syndrome
Hemolytic Uremic Syndrome (HUS), including fatalities from renal failure or the requirement for dialysis, can occur with Gemcitabine for Injection. In clinical trials, HUS occurred in 0.25% of 2429 patients. Most fatal cases of renal failure were due to HUS [see Adverse Reactions ( 6.1)] . Serious cases of thrombotic microangiopathy other than HUS have been reported with Gemcitabine for Injection [see Adverse Reactions ( 6.2)] .
Assess renal function prior to initiation of Gemcitabine for Injection and periodically during treatment. Consider the diagnosis of HUS in patients who develop anemia with evidence of microangiopathic hemolysis; increased bilirubin or LDH; reticulocytosis; severe thrombocytopenia; or renal failure (increased serum creatinine or BUN). Permanently discontinue Gemcitabine for Injection in patients with HUS or severe renal impairment. Renal failure may not be reversible even with the discontinuation of therapy.
5.5 Hepatic Toxicity
Drug-induced liver injury, including liver failure and death, has been reported in patients receiving Gemcitabine for Injection alone or with other potentially hepatotoxic drugs [see Adverse Reactions ( 6.1, 6.2)] . Administration of Gemcitabine for Injection in patients with concurrent liver metastases or a pre-existing medical history of hepatitis, alcoholism, or liver cirrhosis can lead to exacerbation of the underlying hepatic insufficiency. Assess hepatic function prior to initiation of Gemcitabine for Injection and periodically during treatment. Permanently discontinue Gemcitabine for Injection in patients who develop severe hepatic toxicity.
5.6 Embryo-Fetal Toxicity
Based on animal data and its mechanism of action, Gemcitabine for Injection can cause fetal harm when administered to a pregnant woman. Gemcitabine was teratogenic, embryotoxic, and fetotoxic in mice and rabbits.
Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with Gemcitabine for Injection and for 6 months after the final dose. Advise male patients with female partners of reproductive potential to use effective contraception during treatment with Gemcitabine for Injection and for 3 months following the final dose [see Use in Specific Populations ( 8.1, 8.3)] .
5.7 Exacerbation of Radiation Therapy Toxicity
Gemcitabine for Injection is not recommended for use in combination with radiation therapy.
Concurrent (given together or ≤7 days apart)
Life-threatening mucositis, especially esophagitis and pneumonitis occurred in a trial in which Gemcitabine for Injection was administered at a dose of 1000 mg/m 2to patients with non-small cell lung cancer for up to 6 consecutive weeks concurrently with thoracic radiation.
5.8 Capillary Leak Syndrome
Capillary leak syndrome (CLS) with severe consequences has been reported in patients receiving Gemcitabine for Injection as a single agent or in combination with other chemotherapeutic agents [see Adverse Reactions ( 6.2)] . Permanently discontinue Gemcitabine for Injection if CLS develops during therapy.
5.9 Posterior Reversible Encephalopathy Syndrome
Posterior reversible encephalopathy syndrome (PRES) has been reported in patients receiving Gemcitabine for Injection as a single agent or in combination with other chemotherapeutic agents [see Adverse Reactions ( 6.2)] . PRES can present with headache, seizure, lethargy, hypertension, confusion, blindness, and other visual and neurologic disturbances. Confirm the diagnosis of PRES with magnetic resonance imaging (MRI). Permanently discontinue Gemcitabine for Injection if PRES develops during therapy.
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Hypersensitivity [see Contraindications ( 4)]
- Schedule-Dependent Toxicity [see Warnings and Precautions ( 5.1)]
- Myelosuppression [see Warnings and Precautions ( 5.2)]
- Pulmonary Toxicity and Respiratory Failure [see Warnings and Precautions ( 5.3)]
- Hemolytic Uremic Syndrome [see Warnings and Precautions ( 5.4)]
- Hepatic Toxicity [see Warnings and Precautions ( 5.5)]
- Exacerbation of Radiation Therapy Toxicity [see Warnings and Precautions ( 5.7)]
- Capillary Leak Syndrome [see Warnings and Precautions ( 5.8)]
- Posterior Reversible Encephalopathy Syndrome [see Warnings and Precautions ( 5.9)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Single Agent
The data described below reflect exposure to Gemcitabine for Injection as a single agent administered at doses between 800 mg/m 2to 1250 mg/m 2intravenously over 30 minutes once weekly in 979 patients with various malignancies. The most common (≥20%) adverse reactions of single agent Gemcitabine for Injection are nausea/vomiting, anemia, increased alanine aminotransferase (ALT), increased aspartate aminotransferase (AST), neutropenia, increased alkaline phosphatase, proteinuria, fever, hematuria, rash, thrombocytopenia, dyspnea, and edema. The most common (≥5%) Grade 3 or 4 adverse reactions were neutropenia, nausea/vomiting, increased ALT, increased alkaline phosphatase, anemia, increased AST, and thrombocytopenia. Approximately 10% of the 979 patients discontinued Gemcitabine for Injection due to adverse reactions. Adverse reactions resulting in discontinuation of Gemcitabine for Injection in 2% of 979 patients were cardiovascular adverse reactions (myocardial infarction, cerebrovascular accident, arrhythmia, and hypertension) and adverse reactions resulting in discontinuation of Gemcitabine for Injection in <1% of 979 patients were anemia, thrombocytopenia, hepatic dysfunction, renal dysfunction, nausea/vomiting, fever, rash, dyspnea, hemorrhage, infection, stomatitis, somnolence, flu-like syndrome, and edema.
Tables 5and 6present the incidence of selected adverse reactions and laboratory abnormalities reported in patients with various malignancies receiving single agent Gemcitabine for Injection across 5 clinical trials. Additional clinically significant adverse reactions are provided following Table 6.
|
aGrade based on criteria from the World Health Organization (WHO). |
|||
|
bFor approximately 60% of patients, non-laboratory adverse reactions were graded only if assessed to be possibly drug-related. |
|||
|
cN=699-974; all patients with laboratory or non-laboratory data. |
|||
| Adverse Reactionsb | Gemcitabine for Injectionc | ||
| All Grades
(%) | Grade 3
(%) | Grade 4
(%) |
|
| Nausea and Vomiting | 69 | 13 | 1 |
| Fever | 41 | 2 | 0 |
| Rash | 30 | <1 | 0 |
| Dyspnea | 23 | 3 | <1 |
| Diarrhea | 19 | 1 | 0 |
| Hemorrhage | 17 | <1 | <1 |
| Infection | 16 | 1 | <1 |
| Alopecia | 15 | <1 | 0 |
| Stomatitis | 11 | <1 | 0 |
| Somnolence | 11 | <1 | <1 |
| Paresthesias | 10 | <1 | 0 |
|
aGrade based on criteria from the WHO. |
|||
|
bRegardless of causality. |
|||
|
cN=699-974; all patients with laboratory or non-laboratory data. |
|||
| Laboratory Abnormalityb | Gemcitabine for Injectionc | ||
| All Grades
(%) | Grade 3
(%) | Grade 4
(%) |
|
| Hematologic | |||
| Anemia | 68 | 7 | 1 |
| Neutropenia | 63 | 19 | 6 |
| Thrombocytopenia | 24 | 4 | 1 |
| Hepatic | |||
| Increased ALT | 68 | 8 | 2 |
| Increased AST | 67 | 6 | 2 |
| Increased Alkaline Phosphatase | 55 | 7 | 2 |
| Hyperbilirubinemia | 13 | 2 | <1 |
| Renal | |||
| Proteinuria | 45 | <1 | 0 |
| Hematuria | 35 | <1 | 0 |
| Increased BUN | 16 | 0 | 0 |
| Increased Creatinine | 8 | <1 | 0 |
Additional adverse reactions include the following:
- Transfusion requirements: Red blood cell transfusions (19%); platelet transfusions (<1%)
- Edema: Edema (13%), peripheral edema (20%), generalized edema (<1%)
- Flu-like symptoms: Fever, asthenia, anorexia, headache, cough, chills, myalgia, asthenia insomnia, rhinitis, sweating and/or malaise (19%)
- Infection: Sepsis (<1%)
- Extravasation: Injection-site reactions (4%)
- Allergic: Bronchospasm (<2%); anaphylactoid reactions
Ovarian Cancer
Tables 7and 8present the incidence of selected adverse reactions and laboratory abnormalities, occurring in ≥10% of Gemcitabine for Injection-treated patients and at a higher incidence in the Gemcitabine for Injection with carboplatin arm, reported in a randomized trial (Study 1) of Gemcitabine for Injection with carboplatin (n=175) compared to carboplatin alone (n=174) for the second-line treatment of ovarian cancer in women with disease that had relapsed more than 6 months following first-line platinum-based chemotherapy [see Clinical Studies ( 14.1)] . Additional clinically significant adverse reactions, occurring in <10% of patients, are provided following Table 8.
The proportion of patients with dose adjustments for carboplatin (1.8% versus 3.8%), doses of carboplatin omitted (0.2% versus 0) and discontinuing treatment for adverse reactions (11% versus 10%), were similar between arms. Dose adjustment for Gemcitabine for Injection occurred in 10% of patients and Gemcitabine for Injection dose was omitted in 14% of patients in the Gemcitabine for Injection/carboplatin arm.
|
aGrade based on National Cancer Institute CTC Version 2.0. |
||||||
|
bRegardless of causality. |
||||||
|
Adverse Reactionsb | Gemcitabine for Injection/Carboplatin
(N=175) | Carboplatin
(N=174) |
||||
| All Grades
(%) | Grade 3
(%) | Grade 4
(%) | All Grades
(%) | Grade 3
(%) | Grade 4
(%) |
|
| Nausea | 69 | 6 | 0 | 61 | 3 | 0 |
| Alopecia | 49 | 0 | 0 | 17 | 0 | 0 |
| Vomiting | 46 | 6 | 0 | 36 | 2 | <1 |
| Constipation | 42 | 6 | 1 | 37 | 3 | 0 |
| Fatigue | 40 | 3 | <1 | 32 | 5 | 0 |
| Diarrhea | 25 | 3 | 0 | 14 | <1 | 0 |
| Stomatitis/Pharyngitis | 22 | <1 | 0 | 13 | 0 | 0 |
|
aGrade based on National Cancer Institute CTC Version 2.0. |
||||||
|
bRegardless of causality. |
||||||
|
cPercent of patients receiving transfusions. Transfusions are not CTC-graded events. Blood transfusions included both packed red blood cells and whole blood. |
||||||
|
Laboratory Abnormalityb | Gemcitabine for Injection/Carboplatin
(N=175) | Carboplatin
(N=174) |
||||
| All Grades
(%) | Grade 3
(%) | Grade 4
(%) | All Grades
(%) | Grade 3
(%) | Grade 4
(%) |
|
| Hematologic | ||||||
| Neutropenia | 90 | 42 | 29 | 58 | 11 | 1 |
| Anemia | 86 | 22 | 6 | 75 | 9 | 2 |
| Thrombocytopenia | 78 | 30 | 5 | 57 | 10 | 1 |
| RBC Transfusions
c
Platelet Transfusions c | 38
9 | -
- | -
- | 15
3 | -
- | -
- |
Hematopoietic growth factors were administered more frequently in the Gemcitabine for Injection-containing arm: leukocyte growth factor (24% and 10%) and erythropoiesis-stimulating agent (7% and 3.9%).
The following clinically relevant Grade 3 and 4 adverse reactions occurred more frequently in the Gemcitabine for Injection with carboplatin arm: dyspnea (3.4% versus 2.9%), febrile neutropenia (1.1% versus 0), hemorrhagic event (2.3% versus 1.1 %), motor neuropathy (1.1% versus 0.6%), and rash/desquamation (0.6% versus 0).
Breast Cancer
Tables 9and 10present the incidence of selected adverse reactions and laboratory abnormalities, occurring in ≥10% of Gemcitabine for Injection-treated patients and at a higher incidence in the Gemcitabine for Injection with paclitaxel arm, reported in a randomized trial (Study 2) of Gemcitabine for Injection with paclitaxel (n=262) compared to paclitaxel alone (n=259) for the first-line treatment of metastatic breast cancer (MBC) in women who received anthracycline-containing chemotherapy in the adjuvant/neo-adjuvant setting or for whom anthracyclines were contraindicated [see Clinical Studies ( 14.2)] . Additional clinically significant adverse reactions, occurring in <10% of patients, are provided following Table 10.
The requirement for dose reduction of paclitaxel were higher for patients in the Gemcitabine for Injection/paclitaxel arm (5% versus 2%). The number of paclitaxel doses omitted (<1%), the proportion of patients discontinuing treatment for adverse reactions (7% versus 5%) and the number of treatment-related deaths (1 patient in each arm) were similar between the two arms.
|
aGrade based on National Cancer Institute CTC Version 2.0. |
||||||
|
bNon-laboratory events were graded only if assessed to be possibly drug-related. |
||||||
|
Adverse Reactionsb | Gemcitabine for Injection/Paclitaxel
(N=262) | Paclitaxel
(N=259) |
||||
| All Grades
(%) | Grade 3
(%) | Grade 4
(%) | All Grades
(%) | Grade 3
(%) | Grade 4
(%) |
|
| Alopecia | 90 | 14 | 4 | 92 | 19 | 3 |
| Neuropathy-Sensory | 64 | 5 | <1 | 58 | 3 | 0 |
| Nausea | 50 | 1 | 0 | 31 | 2 | 0 |
| Fatigue | 40 | 6 | <1 | 28 | 1 | <1 |
| Vomiting | 29 | 2 | 0 | 15 | 2 | 0 |
| Diarrhea | 20 | 3 | 0 | 13 | 2 | 0 |
| Anorexia | 17 | 0 | 0 | 12 | <1 | 0 |
| Neuropathy-Motor | 15 | 2 | <1 | 10 | <1 | 0 |
| Stomatitis/Pharyngitis | 13 | 1 | <1 | 8 | <1 | 0 |
| Fever | 13 | <1 | 0 | 3 | 0 | 0 |
| Rash/Desquamation | 11 | <1 | <1 | 5 | 0 | 0 |
| Febrile Neutropenia | 6 | 5 | <1 | 2 | 1 | 0 |
|
aGrade based on National Cancer Institute CTC Version 2.0. |
||||||
|
bRegardless of causality. |
||||||
|
Laboratory Abnormalityb | Gemcitabine for Injection/Paclitaxel
(N=262) | Paclitaxel
(N=259) |
||||
| All Grades
(%) | Grade 3
(%) | Grade 4
(%) | All Grades
(%) | Grade 3
(%) | Grade 4
(%) |
|
| Hematologic | ||||||
| Anemia | 69 | 6 | 1 | 51 | 3 | <1 |
| Neutropenia | 69 | 31 | 17 | 31 | 4 | 7 |
| Thrombocytopenia | 26 | 5 | <1 | 7 | <1 | <1 |
| Hepatobiliary | ||||||
| Increased ALT | 18 | 5 | <1 | 6 | <1 | 0 |
| Increased AST | 16 | 2 | 0 | 5 | <1 | 0 |
Clinically relevant Grade 3 or 4 dyspnea occurred with a higher incidence in the Gemcitabine for Injection with paclitaxel arm compared with the paclitaxel arm (1.9% versus 0).
Non-Small Cell Lung Cancer
Tables 11and 12present the incidence of selected adverse reactions and laboratory abnormalities occurring in ≥10% of Gemcitabine for Injection-treated patients and at a higher incidence in the Gemcitabine for Injection with cisplatin arm, reported in a randomized trial (Study 3) of Gemcitabine for Injection with cisplatin (n=260) administered in 28-day cycles as compared to cisplatin alone (n=262) in patients receiving first-line treatment for locally advanced or metastatic NSCLC [see Clinical Studies ( 14.3)] .
Patients randomized to Gemcitabine for Injection with cisplatin received a median of 4 cycles of treatment and those randomized to cisplatin alone received a median of 2 cycles of treatment. In this trial, the requirement for dose adjustments (>90% versus 16%), discontinuation of treatment for adverse reactions (15% versus 8%), and the proportion of patients hospitalized (36% versus 23%) were all higher for patients receiving Gemcitabine for Injection with cisplatin compared to those receiving cisplatin alone. The incidence of febrile neutropenia (3% versus<1%), sepsis (4% versus 1%), Grade 3 cardiac dysrhythmias (3% versus <1%) were all higher in the Gemcitabine for Injection with cisplatin arm compared to the cisplatin alone arm. The two-drug combination was more myelosuppressive with 4 (1.5%) possibly treatment-related deaths, including 3 resulting from myelosuppression with infection and one case of renal failure associated with pancytopenia and infection. No deaths due to treatment were reported on the cisplatin arm.
|
aGrade based on National Cancer Institute Common Toxicity Criteria (CTC). |
||||||
|
bNon-laboratory events were graded only if assessed to be possibly drug-related. |
||||||
|
cN=217-253; all Gemcitabine for Injection/cisplatin patients with laboratory or non-laboratory data |
||||||
|
dN=213-248; all cisplatin patients with laboratory or non-laboratory data |
||||||
| Adverse Reactionsb | Gemcitabine for Injection/Cisplatinc | Cisplatind | ||||
| All Grades
(%) | Grade 3
(%) | Grade 4
(%) | All Grades
(%) | Grade 3
(%) | Grade 4
(%) |
|
| Nausea | 93 | 25 | 2 | 87 | 20 | <1 |
| Vomiting | 78 | 11 | 12 | 71 | 10 | 9 |
| Alopecia | 53 | 1 | 0 | 33 | 0 | 0 |
| Neuro Motor | 35 | 12 | 0 | 15 | 3 | 0 |
| Diarrhea | 24 | 2 | 2 | 13 | 0 | 0 |
| Neuro Sensory | 23 | 1 | 0 | 18 | 1 | 0 |
| Infection | 18 | 3 | 2 | 12 | 1 | 0 |
| Fever | 16 | 0 | 0 | 5 | 0 | 0 |
| Neuro Cortical | 16 | 3 | 1 | 9 | 1 | 0 |
| Neuro Mood | 16 | 1 | 0 | 10 | 1 | 0 |
| Local
Neuro Headache | 15
14 | 0
0 | 0
0 | 6
7 | 0
0 | 0
0 |
| Stomatitis | 14 | 1 | 0 | 5 | 0 | 0 |
| Hemorrhage | 14 | 1 | 0 | 4 | 0 | 0 |
| Hypotension | 12 | 1 | 0 | 7 | 1 | 0 |
| Rash | 11 | 0 | 0 | 3 | 0 | 0 |
|
aGrade based on National Cancer Institute CTC. |
||||||
|
bRegardless of causality. |
||||||
|
cN=217-253; all Gemcitabine for Injection/cisplatin patients with laboratory or non-laboratory data |
||||||
|
dN=213-248; all cisplatin patients with laboratory or non-laboratory data |
||||||
|
ePercent of patients receiving transfusions. Percent transfusions are not CTC-graded events. |
||||||
| Laboratory Abnormalityb | Gemcitabine for Injection/Cisplatinc | Cisplatind | ||||
| All Grades
(%) | Grade 3
(%) | Grade 4
(%) | All Grades
(%) | Grade 3
(%) | Grade 4
(%) |
|
| Hematologic | ||||||
| Anemia | 89 | 22 | 3 | 67 | 6 | 1 |
| Thrombocytopenia | 85 | 25 | 25 | 13 | 3 | 1 |
| Neutropenia | 79 | 22 | 35 | 20 | 3 | 1 |
| Lymphopenia
RBC Transfusions e Platelet Transfusions e | 75
39 21 | 25
- - | 18
- - | 51
13 <1 | 12
- - | 5
- - |
| Hepatic | ||||||
| Increased Transaminases | 22 | 2 | 1 | 10 | 1 | 0 |
| Increased Alkaline Phosphatase | 19 | 1 | 0 | 13 | 0 | 0 |
| Renal
Increased Creatinine |
38 |
4 |
<1 |
31 |
2 |
<1 |
| Proteinuria | 23 | 0 | 0 | 18 | 0 | 0 |
| Hematuria | 15 | 0 | 0 | 13 | 0 | 0 |
| Other Laboratory | ||||||
| Hyperglycemia | 30 | 4 | 0 | 23 | 3 | 0 |
| Hypomagnesemia | 30 | 4 | 3 | 17 | 2 | 0 |
| Hypocalcemia | 18 | 2 | 0 | 7 | 0 | <1 |
Tables 13and 14present the incidence of selected adverse reactions and laboratory abnormalities occurring in ≥10% of Gemcitabine for Injection-treated patients and at a higher incidence in the Gemcitabine for Injection with cisplatin arm, reported in a randomized trial (Study 4) of Gemcitabine for Injection with cisplatin (n=69) administered in 21-day cycles as compared to etoposide with cisplatin (n=66) in patients receiving first-line treatment for locally advanced or metastatic NSCLC [see Clinical Studies ( 14.3)] . Additional clinically significant adverse reactions are provided following Table 14.
Patients in the Gemcitabine for Injection/cisplatin (GC) arm received a median of 5 cycles and those in the etoposide/cisplatin (EC) arm received a median of 4 cycles. The majority of patients receiving more than one cycle of treatment required dose adjustments; 81% in the GC arm and 68% in the EC arm. The incidence of hospitalizations for adverse reactions was 22% in the GC arm and 27% in the EC arm. The proportion of patients who discontinued treatment for adverse reactions was higher in the GC arm (14% versus 8%). The proportion of patients who were hospitalized for febrile neutropenia was lower in the GC arm (7% versus 12%). There was one death attributed to treatment, a patient with febrile neutropenia and renal failure, which occurred in the GC arm.
|
aGrade based on criteria from the WHO. |
||||||
|
bNon-laboratory events were graded only if assessed to be possibly drug-related. Pain data were not collected. |
||||||
|
cN=67-69; all Gemcitabine for Injection/cisplatin patients with laboratory or non-laboratory data. |
||||||
|
dN=57-63; all Etoposide/cisplatin patients with laboratory or non-laboratory data. |
||||||
|
eFlu-like syndrome and edema were not graded. |
||||||
|
Adverse Reactionsb | Gemcitabine for Injection/Cisplatinc | Etoposide/Cisplatind | ||||
| All Grades
(%) | Grade 3
(%) | Grade 4
(%) | All Grades
(%) | Grade 3
(%) | Grade 4
(%) |
|
| Nausea and Vomiting | 96 | 35 | 4 | 86 | 19 | 7 |
| Alopecia | 77 | 13 | 0 | 92 | 51 | 0 |
| Paresthesias | 38 | 0 | 0 | 16 | 2 | 0 |
| Infection | 28 | 3 | 1 | 21 | 8 | 0 |
| Stomatitis | 20 | 4 | 0 | 18 | 2 | 0 |
| Diarrhea | 14 | 1 | 1 | 13 | 0 | 2 |
| Edema e | 12 | - | - | 2 | - | - |
| Rash | 10 | 0 | 0 | 3 | 0 | 0 |
| Hemorrhage | 9 | 0 | 3 | 3 | 0 | 3 |
| Fever | 6 | 0 | 0 | 3 | 0 | 0 |
| Somnolence | 3 | 0 | 0 | 3 | 2 | 0 |
| Flu-like Syndrome e | 3 | - | - | 0 | - | - |
| Dyspnea | 1 | 0 | 1 | 3 | 0 | 0 |
|
aGrade based on criteria from the WHO. |
||||||
|
bRegardless of causality. |
||||||
|
cN=67-69; all Gemcitabine for Injection/cisplatin patients with laboratory or non-laboratory data. |
||||||
|
dN=57-63; all Etoposide/cisplatin patients with laboratory or non-laboratory data. |
||||||
|
eWHO grading scale not applicable to proportion of patients with transfusions. |
||||||
| Laboratory Abnormalityb | Gemcitabine for Injection/Cisplatinc | Etoposide/Cisplatind | ||||
| All Grades
(%) | Grade 3
(%) | Grade 4
(%) | All Grades
(%) | Grade 3
(%) | Grade 4
(%) |
|
| Hematologic | ||||||
| Anemia | 88 | 22 | 0 | 77 | 13 | 2 |
| Neutropenia | 88 | 36 | 28 | 87 | 20 | 56 |
| Thrombocytopenia | 81 | 39 | 16 | 45 | 8 | 5 |
| RBC Transfusions e | 29 | - | - | 21 | - | - |
| Platelet Transfusions e | 3 | - | - | 8 | - | - |
| Hepatic | ||||||
| Increased Alkaline Phosphatase | 16 | 0 | 0 | 11 | 0 | 0 |
| Increased ALT | 6 | 0 | 0 | 12 | 0 | 0 |
| Increased AST | 3 | 0 | 0 | 11 | 0 | 0 |
| Renal | ||||||
| Hematuria | 22 | 0 | 0 | 10 | 0 | 0 |
| Proteinuria | 12 | 0 | 0 | 5 | 0 | 0 |
| Increased BUN | 6 | 0 | 0 | 4 | 0 | 0 |
| Increased Creatinine | 2 | 0 | 0 | 2 | 0 | 0 |
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of Gemcitabine for Injection. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Blood and lymphatic system: Thrombotic microangiopathy (TMA)
- Cardiovascular: Congestive heart failure, myocardial infarction, arrhythmias, supraventricular arrhythmias
- Vascular:Peripheral vasculitis, gangrene, capillary leak syndrome
- Skin:Cellulitis, pseudocellulitis, severe skin reactions, including desquamation and bullous skin eruptions
- Hepatic: Hepatic failure, hepatic veno-occlusive disease
- Pulmonary: Interstitial pneumonitis, pulmonary fibrosis, pulmonary edema, adult respiratory distress syndrome (ARDS), pulmonary eosinophilia
- Nervous System:Posterior reversible encephalopathy syndrome (PRES)
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on animal data and its mechanism of action, Gemcitabine for Injection can cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology ( 12.1)] . There are no available data on the use of Gemcitabine for Injection in pregnant women. In animal reproduction studies, gemcitabine was teratogenic, embryotoxic, and fetotoxic in mice and rabbits (see Data) . Advise pregnant women of the potential risk to a fetus [see Use in Special Populations ( 8.3)] .
In the U.S. general population, the estimated background risk of major birth defects and miscarriages in clinically recognized pregnancies is 2 to 4% and 15 to 20% respectively.
Data
Animal Data
Gemcitabine is embryotoxic in mice. Daily dosing of gemcitabine to pregnant mice increased the incidence of fetal malformation (cleft palate, incomplete ossification) at doses of 1.5 mg/kg/day [approximately 0.005 times the 1000 mg/m 2clinical dose based on body surface area (BSA)]. Gemcitabine was embryotoxic and fetotoxic in rabbits. Daily dosing of gemcitabine to pregnant rabbits resulted in fetotoxicity (decreased fetal viability, reduced litter sizes, and developmental delays) and increased the incidence of fetal malformations (fused pulmonary artery, absence of gall bladder) at doses of 0.1 mg/kg/day (approximately 0.002 times the 1000 mg/m 2clinical dose based on BSA).
8.2 Lactation
Risk Summary
There is no information regarding the presence of Gemcitabine for Injection or its metabolites in human milk, or their effects on the breastfed infant or on milk production. Due to the potential for serious adverse reactions in breastfed infants from Gemcitabine for Injection, advise women not to breastfeed during treatment with Gemcitabine for Injection and for at least one week following the last dose.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Verify pregnancy status in females of reproductive potential prior to initiating Gemcitabine for Injection [see Use in Specific Populations ( 8.1)] .
Contraception
Gemcitabine for Injection can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations ( 8.1)].
Females
Because of the potential for genotoxicity, advise females of reproductive potential to use effective contraception during treatment with Gemcitabine for Injection and for 6 months after the final dose of Gemcitabine for Injection.
Males
Because of the potential for genotoxicity, advise males with female partners of reproductive potential to use effective contraception during treatment with Gemcitabine for Injection and for 3 months after the final dose [see Nonclinical Toxicology ( 13.1)] .
Infertility
Males
Based on animal studies, Gemcitabine for Injection may impair fertility in males of reproductive potential [see Nonclinical Toxicology ( 13.1)] . It is not known whether these effects on fertility are reversible.
8.4 Pediatric Use
The safety and effectiveness of Gemcitabine for Injection have not been established in pediatric patients.
The safety and pharmacokinetics of gemcitabine were evaluated in a trial in pediatric patients with refractory leukemia. The maximum tolerated dose was 10 mg/m 2/min for 360 minutes weekly for three weeks followed by a one-week rest period.
The safety and activity of Gemcitabine for Injection were evaluated in a trial of pediatric patients with relapsed acute lymphoblastic leukemia (22 patients) and acute myelogenous leukemia (10 patients) at a dose of 10 mg/m 2/min administered over 360 minutes weekly for three weeks followed by a one-week rest period. Patients with M1 or M2 bone marrow on Day 28 who did not experience unacceptable toxicity were eligible to receive a maximum of one additional four-week course. Toxicities observed included myelosuppression, febrile neutropenia, increased serum transaminases, nausea, and rash/desquamation. No meaningful clinical activity was observed in this trial.
8.5 Geriatric Use
In clinical studies which enrolled 979 patients with various malignancies who received single agent Gemcitabine for Injection, no overall differences in safety were observed between patients aged 65 and older and younger patients, with the exception of a higher rate of Grade 3-4 thrombocytopenia in older patients as compared to younger patients.
In a randomized trial in women with ovarian cancer (Study 1), 175 women received Gemcitabine for Injection with carboplatin, of which 29% were age 65 years or older. Similar effectiveness was observed between older and younger women. There was significantly higher Grade 3-4 neutropenia in women 65 years of age or older [see Dosage and Administration ( 2.1)] .
Gemcitabine for Injection clearance is affected by age; however, there are no recommended dose adjustments based on patients' age [see Clinical Pharmacology ( 12.3)].
8.6 Gender
Gemcitabine for Injection clearance is decreased in females [see Clinical Pharmacology ( 12.3)] . In single agent studies of Gemcitabine for Injection, women, especially older women, were more likely not to proceed to a subsequent cycle and to experience Grade 3-4 neutropenia and thrombocytopenia [see Dosage and Administration ( 2.1, 2.2, 2.3, 2.4)] .
10 OVERDOSAGE
There is no known antidote for overdoses of gemcitabine. Myelosuppression, paresthesias, and severe rash were the principal toxicities seen when a single dose as high as 5700 mg/m 2was administered by intravenous infusion over 30 minutes every 2 weeks to several patients in a dose-escalation study. In the event of suspected overdose, monitor with appropriate blood counts and provide supportive therapy, as necessary.
11 DESCRIPTION
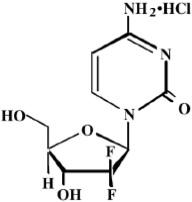
Gemcitabine is a nucleoside metabolic inhibitor. Gemcitabine hydrochloride is 2'-deoxy-2',2'-difluorocytidine monohydrochloride (β-isomer) with the following structural formula:
The empirical formula for gemcitabine hydrochloride is C 9H 11F 2N 3O 4• HCl. It has a molecular weight of 299.66 g/mol.
Gemcitabine hydrochloride is soluble in water, slightly soluble in methanol, and practically insoluble in ethanol and polar organic solvents.
Gemcitabine for Injection, USP is a sterile white to off-white lyophilized powder and available as 200 mg and 1 gram single-dose vials for intravenous use only. Each 200 mg vial contains 200 mg gemcitabine (equivalent to 227.7 mg gemcitabine hydrochloride), 200 mg mannitol and 12.5 mg sodium acetate. Each 1 gram vial contains 1 gram gemcitabine (equivalent to 1.139 gram gemcitabine hydrochloride), 1 gram mannitol, and 62.5 mg sodium acetate. Hydrochloric acid and/or sodium hydroxide may have been added for pH adjustment.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Gemcitabine kills cells undergoing DNA synthesis and blocks the progression of cells through the G1/S-phase boundary. Gemcitabine is metabolized by nucleoside kinases to diphosphate (dFdCDP) and triphosphate (dFdCTP) nucleosides. Gemcitabine diphosphate inhibits ribonucleotide reductase, an enzyme responsible for catalyzing the reactions that generate deoxynucleoside triphosphates for DNA synthesis, resulting in reductions in deoxynucleotide concentrations, including dCTP. Gemcitabine triphosphate competes with dCTP for incorporation into DNA. The reduction in the intracellular concentration of dCTP by the action of the diphosphate enhances the incorporation of gemcitabine triphosphate into DNA (self-potentiation). After the gemcitabine nucleotide is incorporated into DNA, only one additional nucleotide is added to the growing DNA strands, which eventually results in the initiation of apoptotic cell death.
12.3 Pharmacokinetics
The pharmacokinetics of gemcitabine were examined in 353 patients with various solid tumors. Pharmacokinetic parameters were derived using data from patients treated for varying durations of therapy given weekly with periodic rest weeks and using both short infusions (<70 minutes) and long infusions (70 to 285 minutes). The total Gemcitabine for Injection dose varied from 500 mg/m 2to 3600 mg/m 2.
Distribution
The volume of distribution was increased with infusion length. Volume of distribution of gemcitabine was 50 L/m 2following infusions lasting <70 minutes. For long infusions, the volume of distribution rose to 370 L/m 2.
Gemcitabine pharmacokinetics are linear and are described by a 2-compartment model. Population pharmacokinetic analyses of combined single and multiple dose studies showed that the volume of distribution of gemcitabine was significantly influenced by duration of infusion and sex. Gemcitabine plasma protein binding is negligible.
Elimination
Metabolism
The active metabolite, gemcitabine triphosphate, can be extracted from peripheral blood mononuclear cells. The half-life of the terminal phase for gemcitabine triphosphate from mononuclear cells ranges from 1.7 to 19.4 hours.
Excretion
Gemcitabine disposition was studied in 5 patients who received a single 1000 mg/m 2of radiolabeled drug as a 30-minute infusion. Within one week, 92% to 98% of the dose was recovered, almost entirely in the urine. Gemcitabine (<10%) and the inactive uracil metabolite, 2′-deoxy-2′,2′-difluorouridine (dFdU) accounted for 99% of the excreted dose. The metabolite dFdU is also found in plasma.
Specific Populations
Geriatric Patients
Clearance of gemcitabine was affected by age. The lower clearance in geriatric patients results in higher concentrations of gemcitabine for any given dose. Differences in either clearance or volume of distribution based on patient characteristics or the duration of infusion result in changes in half-life and plasma concentrations. Table 15shows plasma clearance and half-life of gemcitabine following short infusions for typical patients by age and sex.
|
aHalf-life for patients receiving a <70 minute infusion. |
||||
| Age | Clearance Men
(L/hr/m2) | Clearance Women
(L/hr/m2) | Half-LifeaMen
(min) | Half-LifeaWomen
(min) |
| 29 | 92.2 | 69.4 | 42 | 49 |
| 45 | 75.7 | 57.0 | 48 | 57 |
| 65 | 55.1 | 41.5 | 61 | 73 |
| 79 | 40.7 | 30.7 | 79 | 94 |
Gemcitabine half-life for short infusions ranged from 42 to 94 minutes and for long infusions varied from 245 to 638 minutes, depending on age and sex, reflecting a greatly increased volume of distribution with longer infusions.
Male and Female Patients
Females have lower clearance and longer half-lives than male patients as described in Table 15.
Drug Interaction Studies
When Gemcitabine for Injection (1250 mg/m 2on Days 1 and 8) and cisplatin (75 mg/m 2on Day 1) were administered in patients with NSCLC, the clearance of gemcitabine on Day 1 was 128 L/hr/m 2and on Day 8 was 107 L/hr/m 2. Data from patients with NSCLC demonstrate that Gemcitabine for Injection and carboplatin given in combination does not alter the pharmacokinetics of gemcitabine or carboplatin compared to administration of either single agent; however, due to wide confidence intervals and small sample size, interpatient variability may be observed.
Data from metastatic breast cancer patients shows that Gemcitabine for Injection has little or no effect on the pharmacokinetics (clearance and half-life) of paclitaxel and paclitaxel has little or no effect on the pharmacokinetics of gemcitabine.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal studies to evaluate the carcinogenic potential of Gemcitabine for Injection have not been conducted. Gemcitabine was mutagenic in an in vitromouse lymphoma (L5178Y) assay and was clastogenic in an in vivomouse micronucleus assay. Gemcitabine intraperitoneal doses of 0.5 mg/kg/day [about 1/700 the 1000 mg/m 2clinical dose based on body surface area (BSA)] in male mice resulted in moderate to severe hypospermatogenesis, decreased fertility, and decreased implantations. In female mice, fertility was not affected but maternal toxicities were observed at 1.5 mg/kg/day administered intravenously (about 1/200 the 1000 mg/m 2clinical dose based on BSA) and fetotoxicity or embryolethality was observed at 0.25 mg/kg/day administered intravenously (about 1/1300 the 1000 mg/m 2clinical dose based on BSA).
14 CLINICAL STUDIES
14.1 Ovarian Cancer
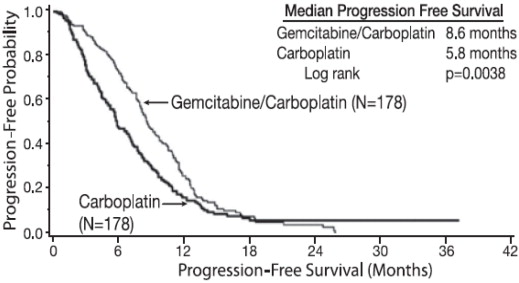
The efficacy of Gemcitabine for Injection was evaluated in a randomized trial (Study 1) conducted in women with advanced ovarian cancer that had relapsed at least 6 months after first-line platinum-based therapy. Patients were randomized to receive either Gemcitabine for Injection 1000 mg/m 2on Days 1 and 8 of each 21-day cycle with carboplatin AUC 4 on Day 1 after Gemcitabine for Injection administration (n=178) or carboplatin AUC 5 on Day 1 of each 21-day cycle (n=178). The major efficacy outcome measure was progression-free survival (PFS).
A total of 356 patients were enrolled. Demographics and baseline characteristics are shown in Table 16.
Efficacy results are presented in Table 17and Figure 1. The addition of Gemcitabine for Injection to carboplatin resulted in statistically significant improvements in PFS and overall response rate. Approximately 75% of patients in each arm received additional chemotherapy for disease progression; 13 of 120 patients in the carboplatin alone arm received Gemcitabine for Injection for treatment of disease progression. There was no significant difference in overall survival between the treatment arms.
|
a5 patients on Gemcitabine for Injection with carboplatin arm and 4 patients on carboplatin arm had no baseline Eastern Cooperative Oncology Group (ECOG) performance status. |
||
|
b2 patients on Gemcitabine for Injection with carboplatin arm and 1 patient on carboplatin arm had platinum-free interval <6 months. |
||
| Gemcitabine for Injection/Carboplatin
(N=178) | Carboplatin
(N=178) |
|
| Median age, years
Range | 59
36 to 78 | 58
21 to 81 |
| Baseline ECOG performance status 0-1 a | 94% | 95% |
| Disease Status
Evaluable Bidimensionally measurable |
8% 92% |
3% 96% |
| Platinum-free interval
b
6-12 months >12 months |
40% 59% |
40% 60% |
| First-line therapy
Platinum-taxane combination Platinum-non-taxane combination Platinum monotherapy |
70% 29% 1% |
71% 28% 1% |
|
aCI=confidence interval. |
||
|
bLog rank, unadjusted. |
||
|
cChi square. |
||
|
dCR=Complete response. |
||
|
ePR with PRNM=Partial response with partial response, non-measurable disease. |
||
|
fIndependently reviewed cohort – Gemcitabine for Injection/carboplatin (n=121), carboplatin (n=101); independent reviewers unable to measure disease detected by sonography or physical exam. |
||
| Efficacy Parameter | Gemcitabine for Injection/Carboplatin
(N=178) | Carboplatin
(N=178) |
| Progression-Free Survival | ||
| Median (95% CI a) in months | 8.6 (8.0, 9.7) | 5.8 (5.2, 7.1) |
| Hazard Ratio (95% CI) | 0.72 (0.57, 0.90) | |
| p-value b | p=0.0038 | |
| Overall Survival | ||
| Median (95% CI) in months | 18.0 (16.2, 20.3) | 17.3 (15.2, 19.3) |
| Hazard Ratio (95% CI) | 0.98 (0.78, 1.24) | |
| p-value b | p=0.8977 | |
| Overall Response Rate by
Investigator Review | 47.2% | 30.9% |
| p-value c | p=0.0016 | |
| CR d | 14.6% | 6.2% |
| PR with PRNM e | 32.6% | 24.7% |
| Overall Response Ratefby
Independent Review | 46.3% | 35.6% |
| p-value c | p=0.11 | |
| CR d | 9.1% | 4.0% |
| PR with PRNM e | 37.2% | 31.7% |
Figure 1: Kaplan-Meier Curves for Progression-Free Survival in Study 1
14.2 Breast Cancer
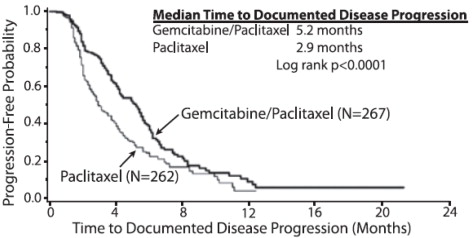
The efficacy of Gemcitabine for Injection was evaluated in a multinational, randomized, open-label trial (Study 2) conducted in women receiving initial treatment for metastatic breast cancer and who have received prior adjuvant/neoadjuvant anthracycline chemotherapy unless clinically contraindicated. Patients were randomized to receive either Gemcitabine for Injection 1250 mg/m 2on Days 1 and 8 of each 21-day cycle with paclitaxel 175 mg/m 2administered on Day 1 before Gemcitabine for Injection administration (n=267) or paclitaxel 175 mg/m 2on Day 1 of each 21-day cycle (n=262). The major efficacy outcome measure was time to documented disease progression.
A total of 529 patients were enrolled. Demographic and baseline characteristics were similar between treatment arms ( Table 18).
Efficacy results are presented in Table 19and Figure 2. The addition of Gemcitabine for Injection to paclitaxel resulted in statistically significant improvement in time to documented disease progression and overall response rate compared to paclitaxel alone. There was no significant difference in overall survival.
|
aKarnofsky Performance Status. |
||
| Gemcitabine for Injection/Paclitaxel
(N=267) | Paclitaxel
(N=262) |
|
| Median age, years
Range | 53
26 to 83 | 52
26 to 75 |
| Metastatic disease | 97% | 97% |
| Baseline KPS a≥90 | 70% | 74% |
| Number of tumor sites
1-2 ≥3 |
57% 43% |
59% 41% |
| Visceral disease | 73% | 73% |
| Prior anthracycline | 97% | 96% |
|
aThese represent reconciliation of investigator and Independent Review Committee assessments according to a predefined algorithm. |
||
|
bBased on the ITT population. |
||
|
Efficacy Parameter | Gemcitabine for Injection/Paclitaxel
(N=267) | Paclitaxel
(N=262) |
| Time to Documented Disease Progressiona | ||
| Median (95% CI) in months | 5.2 (4.2, 5.6) | 2.9 (2.6, 3.7) |
| Hazard Ratio (95% CI) | 0.650 (0.524, 0.805) | |
| p-value | p<0.0001 | |
| Overall Survivalb | ||
| Median (95% CI) in months | 18.6 (16.5, 20.7) | 15.8 (14.1, 17.3) |
| Hazard Ratio (95% CI) | 0.86 (0.71, 1.04) | |
| p-value | Not Significant | |
| Overall Response Rate | 40.8% | 22.1% |
| (95% CI) | (34.9, 46.7) | (17.1, 27.2) |
| p-value | p<0.0001 | |
Figure 2: Kaplan-Meier Curves for Time to Documented Disease Progression in Study 2
14.3 Non-Small Cell Lung Cancer
The efficacy of Gemcitabine for Injection was evaluated in two randomized, multicenter trials.
Study 3: 28-Day Schedule
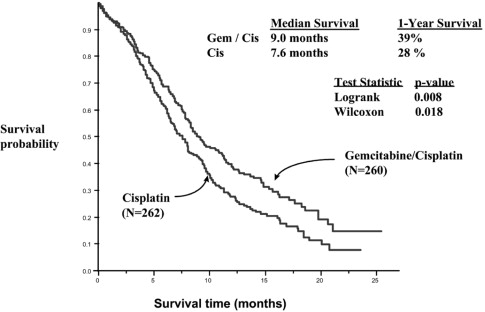
A multinational, randomized trial (Study 3) compared Gemcitabine for Injection with cisplatin to cisplatin alone in the treatment of patients with inoperable Stage IIIA, IIIB, or IV NSCLC who had not received prior chemotherapy. Patients were randomized to receive either Gemcitabine for Injection 1000 mg/m 2on Days 1, 8, and 15 of each 28-day cycle with cisplatin 100 mg/m 2on Day 1 after Gemcitabine for Injection administration (N=260) or cisplatin 100 mg/m 2on Day 1 of each 28-day cycle (N=262). The major efficacy outcome measure was overall survival.
A total of 522 patients were enrolled. Demographics and baseline characteristics ( Table 20) were similar between arms with the exception of histologic subtype of NSCLC, with 48% of patients on the cisplatin arm and 37% of patients on the Gemcitabine for Injection with cisplatin arm having adenocarcinoma.
Study 4: 21-Day Schedule
A randomized (1:1), multicenter trial (Study 4) was conducted in patients with Stage IIIB or IV NSCLC. Patients were randomized to receive either Gemcitabine for Injection 1250 mg/m 2on Days 1 and 8 of each 21-day cycle with cisplatin 100 mg/m 2on Day 1 after Gemcitabine for Injection administration or etoposide 100 mg/m 2intravenously on Days 1, 2, and 3 with cisplatin 100 mg/m 2on Day 1 of each 21-day cycle. The major efficacy outcome measure was response rate.
A total of 135 patients were enrolled. Demographics and baseline characteristics are summarized in Table 20.
Efficacy results are presented in Table 21. There was no significant difference in survival between the two treatment arms. The median survival was 8.7 months for the Gemcitabine for Injection with cisplatin arm versus 7 months for the etoposide with cisplatin arm. Median time to disease progression for the Gemcitabine for Injection with cisplatin arm was 5 months compared to 4.1 months on the etoposide with cisplatin arm (Log rank p=0.015, two-sided). The objective response rate for the Gemcitabine for Injection with cisplatin arm was 33% compared to 14% on the etoposide with cisplatin arm (Fisher's Exact p=0.01, two-sided).
|
aN/A Not applicable. |
||||
|
bKarnofsky Performance Status. |
||||
| Trial | 28-day Schedule (Study 3) | 21-day Schedule (Study 4) | ||
| Gemcitabine for Injection/Cisplatin
(N=260) | Cisplatin
(N=262) | Gemcitabine for Injection/Cisplatin
(N=69) | Etoposide/Cisplatin
(N=66) |
|
| Male
Median age, years Range | 70%
62 36 to 88 | 71%
63 35 to 79 | 93%
58 33 to 76 | 92%
60 35 to 75 |
| Stage IIIA
Stage IIIB Stage IV | 7%
26% 67% | 7%
23% 70% | N/A
a
48% 52% | N/A
a
52% 49% |
| Baseline KPS
b70 to 80
Baseline KPS b90 to 100 | 41%
57% | 44%
55% | 45%
55% | 52%
49% |
|
aCI=confidence intervals. |
||||
|
bp-value two-sided Fisher's Exact test for difference in binomial proportions; log rank test for time-to-event analyses. |
||||
| Trial | 28-day Schedule (Study 3) | 21-day Schedule (Study 4) | ||
| Efficacy Parameter | Gemcitabine for Injection/
Cisplatin (N=260) | Cisplatin
(N=262) | Gemcitabine for Injection/ Cisplatin
(N=69) | Etoposide/Cisplatin
(N=66) |
| Survival | ||||
| Median (95% CI a) in months | 9.0 (8.2, 11.0) | 7.6 (6.6, 8.8) | 8.7 (7.8, 10.1) | 7.0 (6.0, 9.7) |
| p-value f | p=0.008 | p=0.18 | ||
| Time to Disease Progression | ||||
| Median (95% CI a) in months | 5.2 (4.2, 5.7) | 3.7 (3.0, 4.3) | 5.0 (4.2, 6.4) | 4.1 (2.4, 4.5) |
| p-value b | p=0.009 | p=0.015 | ||
| Tumor Response | 26% | 10% | 33% | 14% |
| p-value b | p<0.0001 | p=0.01 | ||
Figure 3: Kaplan-Meier Curves for Overall Survival in Study 3
14.4 Pancreatic Cancer
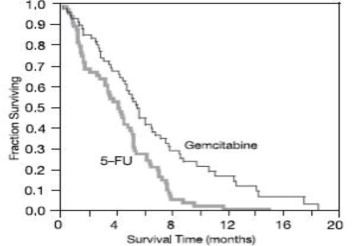
The efficacy of Gemcitabine for Injection was evaluated in two trials (Studies 5 and 6), a randomized, single-blind, two-arm, active-controlled trial (Study 5) conducted in patients with locally advanced or metastatic pancreatic cancer who had received no prior chemotherapy and in a single-arm, open-label, multicenter trial (Study 6) conducted in patients with locally advanced or metastatic pancreatic cancer previously treated with fluorouracil or a fluorouracil-containing regimen. In Study 5, patients were randomized to receive either Gemcitabine for Injection 1000 mg/m 2intravenously over 30 minutes once weekly for 7 weeks followed by a one-week rest, then once weekly for 3 consecutive weeks every 28-days in subsequent cycles (n=63) or fluorouracil 600 mg/m 2intravenously over 30 minutes once weekly (n=63). In Study 6, all patients received Gemcitabine for Injection 1000 mg/m 2intravenously over 30 minutes once weekly for 7 weeks followed by a one-week rest, then once weekly for 3 consecutive weeks every 28-days in subsequent cycles.
The major efficacy outcome measure in both trials was "clinical benefit response". A patient was considered to have had a clinical benefit response if either of the following occurred:
- The patient achieved a ≥50% reduction in pain intensity (Memorial Pain Assessment Card) or analgesic consumption, or a 20-point or greater improvement in performance status (Karnofsky Performance Status) for a period of at least 4 consecutive weeks, without showing any sustained worsening in any of the other parameters. Sustained worsening was defined as 4 consecutive weeks with either any increase in pain intensity or analgesic consumption or a 20-point decrease in performance status occurring during the first 12 weeks of therapy.
OR - The patient was stable on all of the aforementioned parameters and showed a marked, sustained weight gain (≥7% increase maintained for ≥4 weeks) not due to fluid accumulation.
Study 5 enrolled 126 patients. Demographics and baseline characteristics were similar between the arms ( Table 22).
The efficacy results are shown in Table 23and Figure 4. Patients treated with Gemcitabine for Injection had statistically significant increases in clinical benefit response, survival, and time to disease progression compared to those randomized to receive fluorouracil. No confirmed objective tumor responses were observed in either treatment arm.
|
aKarnofsky Performance Status. |
||
| Gemcitabine for Injection
(N=63) | Fluorouracil
(N=63) |
|
| Male | 54% | 54% |
| Median age, years | 62 | 61 |
| Range | 37 to 79 | 36 to 77 |
| Stage IV disease | 71% | 76% |
| Baseline KPS a≤70 | 70% | 68% |
|
ap-value for clinical benefit response calculated using the two-sided test for difference in binomial proportions. All other p-values are calculated using log rank test. |
||
| Efficacy Parameter | Gemcitabine for Injection
(N=63) | Fluorouracil
(N=63) |
| Clinical Benefit Response | 22.2% | 4.8% |
| p-value a | p=0.004 | |
| Overall Survival | ||
| Median (95% CI) in months | 5.7 (4.7, 6.9) | 4.2 (3.1, 5.1) |
| p-value a | p=0.0009 | |
| Time to Disease Progression | ||
| Median (95% CI) in months | 2.1 (1.9, 3.4) | 0.9 (0.9, 1.1) |
| p-value a | p=0.0013 | |
Figure 4: Kaplan-Meier Curves for Overall Survival in Study 5
16 HOW SUPPLIED/STORAGE AND HANDLING
Gemcitabine for Injection, USP is supplied as follows:
| NDC | Gemcitabine for Injection, USP | Package Factor |
| 70860-204-10 | 200 mg Single-Dose Vial | 1 vial per carton |
| 70860-205-50 | 1 gram Single-Dose Vial | 1 vial per carton |
Gemcitabine for Injection, USP is a sterile white to off-white lyophilized powder.
Gemcitabine for Injection, USP is a cytotoxic drug. Follow applicable special handling and disposal procedures. 1
17 PATIENT COUNSELING INFORMATION
Myelosuppression
Advise patients of the risks of myelosuppression. Instruct patients to immediately contact their healthcare provider should any signs or symptoms of infection, including fever, or if bleeding or signs of anemia, occur [see Warnings and Precautions ( 5.2)] .
Pulmonary Toxicity
Advise patients of the risks of pulmonary toxicity, including respiratory failure and death. Instruct patients to immediately contact their healthcare provider for development of shortness of breath, wheezing, or cough [see Warnings and Precautions ( 5.3)] .
Hemolytic Uremic Syndrome and Renal Failure
Advise patients of the risks of hemolytic uremic syndrome and associated renal failure. Instruct patients to immediately contact their healthcare provider for changes in the color or volume of urine output or for increased bruising or bleeding [see Warnings and Precautions ( 5.4)] .
Hepatic Toxicity
Advise patients of the risks of hepatic toxicity including liver failure and death. Instruct patients to immediately contact their healthcare provider for signs of jaundice or for pain/tenderness in the right upper abdominal quadrant [see Warnings and Precautions ( 5.5)] .
Embryo-Fetal Toxicity
Advise females and males of reproductive potential that Gemcitabine for Injection can cause fetal harm. Advise females of reproductive potential to use effective contraception during treatment with Gemcitabine for Injection and for 6 months after the final dose. Advise male patients with female partners of reproductive potential to use effective contraception during treatment with Gemcitabine for Injection and for 3 months after the final dose [see Warnings and Precaution ( 5.6), Use in Specific Populations ( 8.1, 8.3)] .
Lactation
Advise women not to breastfeed during treatment with Gemcitabine for Injection and for at least one week after the last dose [see Use in Specific Populations ( 8.2)] .
| GEMCITABINE
gemcitabine hydrochloride injection, powder, lyophilized, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| GEMCITABINE
gemcitabine hydrochloride injection, powder, lyophilized, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Athenex Pharmaceutical Division, LLC. (080318964) |