PRIMSOL- trimethoprim hydrochloride solution
Avadel CNS Pharmaceuticals, LLC
----------
Primsol® Solution
(trimethoprim hydrochloride oral solution)
Dye-free, alcohol-free, flavored solution,
50 mg trimethoprim per 5 mL
DESCRIPTION
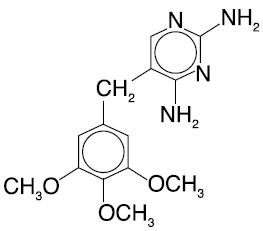
PRIMSOL (trimethoprim hydrochloride oral solution) is a solution of the synthetic antibacterial trimethoprim in water prepared with the aid of hydrochloric acid. Each 5 mL for oral administration contains trimethoprim hydrochloride equivalent to 50 mg trimethoprim and the inactive ingredients bubble gum flavor, fructose, glycerin, methylparaben, monoammonium glycyrrhizinate, povidone, propylparaben, propylene glycol, saccharin sodium, sodium benzoate, sorbitol, water and hydrochloric acid and/or sodium hydroxide to adjust pH to a range of 3.0 - 5.0. Trimethoprim is 2,4-diamino-5-(3,4,5-trimethoxybenzyl) pyrimidine. Trimethoprim is a white to cream-colored, odorless, bitter compound with a molecular formula of C14H18N4O3 and a molecular weight of 290.32 and the following structural formula:
CLINICAL PHARMACOLOGY
Trimethoprim is rapidly absorbed following oral administration. It exists in the blood as unbound, protein-bound and metabolized forms. Ten to twenty percent of trimethoprim is metabolized, primarily in the liver; the remainder is excreted unchanged in the urine. The principal metabolites of trimethoprim are the 1- and 3-oxides and the 3'- and 4'-hydroxy derivatives. The free form is considered to be the therapeutically active form. Approximately 44% of trimethoprim is bound to plasma proteins.
Mean peak plasma concentrations of approximately 1 mcg/mL occur 1 to 4 hours after oral administration of a single 100 mg dose. A single 200 mg dose will result in plasma concentrations approximately twice as high. The mean half-life of trimethoprim is approximately 9 hours. However, patients with severely impaired renal function exhibit an increase in the half-life of trimethoprim, which requires either dosage regimen adjustment or not using the drug in such patients (see DOSAGE AND ADMINISTRATION section). During a 13-week study of trimethoprim tablets administered at a dosage of 50 mg q.i.d., the mean minimum steady-state concentration of the drug was 1.1 mcg/mL. Steady-state concentrations were achieved within two to three days of chronic administration and were maintained throughout the experimental period.
Excretion of trimethoprim is primarily by the kidneys through glomerular filtration and tubular secretion. Urine concentrations of trimethoprim are considerably higher than are the concentrations in the blood. After a single oral dose of 100 mg, urine concentrations of trimethoprim ranged from 30 to 160 mcg/mL during the 0- to 4-hour period and declined to approximately 18 to 91 mcg/mL during the 8- to 24-hour period. A 200 mg single oral dose will result in trimethoprim urine concentrations approximately twice as high. After oral administration, 50% to 60% of trimethoprim is excreted in the urine within 24 hours, approximately 80% of this being unmetabolized trimethoprim.
Trimethoprim half-life, clearance, and volume of distribution vary with age. Excluding newborns, an apparent trend of increasing half-life, volume of distribution, and decreasing clearance is observed with increasing age until adulthood.
Since normal vaginal and fecal flora are the source of most pathogens causing urinary tract infections, it is relevant to consider the distribution of trimethoprim into these sites. Concentrations of trimethoprim in vaginal secretions are consistently greater than those found simultaneously in the serum, being typically 1.6 times the concentrations of simultaneously obtained serum samples. Sufficient trimethoprim is excreted in the feces to markedly reduce or eliminate trimethoprim-susceptible organisms from the fecal flora. The dominant non-Enterobacteriaceae fecal organisms, Bacteroides spp. and Lactobacillus spp., are not susceptible to trimethoprim concentrations obtained with the recommended dosage.
Trimethoprim also concentrates into middle ear fluid (MEF) very efficiently. In a study in children aged 1 to 12 years, administration of a single 4 mg/kg dose resulted in a mean peak MEF concentration of 2.0 mcg/mL.
Trimethoprim also passes the placental barrier and is excreted in breast milk.
Microbiology
Trimethoprim blocks the production of tetrahydrofolic acid from dihydrofolic acid by binding to and reversibly inhibiting the required enzyme, dihydrofolate reductase. This binding is very much stronger for the bacterial enzyme than for the corresponding mammalian enzyme. Thus, trimethoprim selectively interferes with bacterial biosynthesis of nucleic acids and proteins.
Trimethoprim has been shown to be active against most strains of the following microorganisms, both in vitro and in clinical infections as described in the INDICATIONS AND USAGE section.
Aerobic gram-positive microorganisms
| Staphylococcus species | (coagulase-negative strains, including S. saprophyticus) |
| Streptococcus pneumoniae | (penicillin-susceptible strains) |
Aerobic gram-negative microorganisms
| Enterobacter species | |
| Escherichia coli | |
| Haemophilus influenzae | (excluding beta-lactamase negative, ampicillin resistant strains) |
| Klebsiella pneumoniae | |
| Proteus mirabilis |
NOTE: Moraxella catarrhalis isolates were found consistently resistant to trimethoprim.
Susceptibility Tests
Dilution techniques
Quantitative methods are used to determine antimicrobial minimum inhibitory concentrations (MIC's). These MIC's provide estimates of the susceptibility of bacteria to antimicrobial compounds. The MIC's should be determined using a standardized procedure. Standardized procedures are based on a dilution method1 (broth or agar) or equivalent with standardized inoculum concentrations and standardized concentrations of trimethoprim powder. The MIC values should be interpreted according to the following criteria:
For testing aerobic microorganisms isolated from urinary tract infections:
| MIC (mcg/mL) | Interpretation |
|---|---|
| ≤ 8 | Susceptible (S) |
| ≥ 16 | Resistant (R) |
When testing Haemophilus influenzae 1
| MIC (mcg/mL) | Interpretation |
|---|---|
| ≤ 0.5 | Susceptible (S) |
| 1-2 | Intermediate (I) |
| ≥ 4 | Resistant (R) |
When testing Streptococcus pneumoniae 2
| MIC (mcg/mL) | Interpretation |
|---|---|
| ≤ 2 | Susceptible (S) |
| ≥ 4 | Resistant (R) |
A report of "Susceptible" indicates that the pathogen is likely to be inhibited if the antimicrobial compound in the blood reaches the concentrations usually achievable. A report of "Intermediate" indicates that the result should be considered equivocal, and, if the microorganism is not fully susceptible to alternative, clinically feasible drugs, the test should be repeated. This category implies possible clinical applicability in body sites where the drug is physiologically concentrated or in situations where high dosage of drug can be used. This category also provides a buffer zone which prevents small uncontrolled technical factors from causing major discrepancies in interpretation. A report of "Resistant" indicates that the pathogen is not likely to be inhibited if the antimicrobial compound in the blood reaches the concentrations usually achievable; other therapy should be selected.
Standardized susceptibility test procedures require the use of laboratory control microorganisms to control the technical aspects of the laboratory procedures. Standard trimethoprim3 powder should provide the following MIC values:
| Microorganism | MIC (mcg/mL) | |
|---|---|---|
| Escherichia coli | ATCC 25922 | 0.5 - 2 |
| Haemophilus influenzae* | ATCC 49247 | 0.06 - 0.5 |
| Staphylococcus aureus | ATCC 29213 | 1 - 4 |
| Streptococcus pneumoniae† | ATCC 49619 | 1 - 4 |
- 1
- Interpretive criteria applicable only to tests performed by broth microdilution method using Haemophilus Test Medium (HTM).1
- 2
- Interpretive criteria applicable only to tests performed by broth microdilution method using cation-adjusted Mueller-Hinton broth with 2 to 5% lysed horse blood.1
- 3
- Trimethoprim very medium-dependent.
Diffusion techniques
Quantitative methods that require measurement of zone diameters also provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. One such standardized procedure2 requires the use of standardized inoculum concentrations. This procedure uses paper disks impregnated with 5 mcg trimethoprim to test the susceptibility of microorganisms to trimethoprim.
Reports from the laboratory providing results of the standard single-disk susceptibility test with a 5 mcg trimethoprim4 disk should be interpreted according to the following criteria:
For testing aerobic microorganisms isolated from urinary tract infections:
| Zone diameter (mm) | Interpretation |
|---|---|
| ≥16 | Susceptible (S) |
| 11-15 | Intermediate (I) |
| ≤10 | Resistant (R) |
For testing Haemophilus influenzae 5:
| Zone diameter (mm) | Interpretation |
|---|---|
| ≥16 | Susceptible (S) |
| 11-15 | Intermediate (I) |
| ≤10 | Resistant (R) |
Note:
Diffusion techniques are not recommended for determining susceptibility of Streptococcus pneumoniae to trimethoprim.
Interpretation should be as stated above for results using dilution techniques. Interpretation involves correlation of the diameter obtained in the disk test with the MIC for trimethoprim.
As with standardized dilution techniques, diffusion methods require the use of laboratory control microorganisms that are used to control the technical aspects of the laboratory procedures. For the diffusion technique, the 5 mcg trimethoprim4 disk should provide the following zone diameters in this laboratory test quality control strain:
| Microorganism | Zone Diameter (mm) | |
|---|---|---|
|
||
| Escherichia coli | ATCC 25922 | 21 - 28 |
| Haemophilus influenzae* | ATCC 49247 | 27 - 33 |
| Staphylococcus aureus | ATCC 25923 | 19 - 26 |
Note:
Diffusion techniques are not recommended for determining susceptibility of Streptococcus pneumoniae to trimethoprim.
- 4
- Blood-containing media (except for lysed horse blood) are generally not suitable for testing trimethoprim. Mueller-Hinton agar should be checked for excessive levels of thymidine. To determine whether Mueller-Hinton medium has sufficiently low levels of thymidine and thymine, an Enterococcus faecalis (ATCC 29212 or ATCC 33186) may be tested with trimethoprim/sulfamethoxazole disks. A zone of inhibition ≥20 mm that is essentially free of fine colonies indicates a sufficiently low level of thymidine and thymine.
- 5
- Interpretative criteria applicable only to tests performed by disk diffusion method using Haemophilus Test Medium (HTM).2
INDICATIONS AND USAGE
PRIMSOL Solution is indicated for the treatment of infections caused by susceptible strains of the designated microorganisms in the conditions listed below.
Pediatric Patients
Acute Otitis Media
For the treatment of acute otitis media due to susceptible strains of Streptococcus pneumoniae and Haemophilus influenzae.
NOTE: Moraxella catarrhalis isolates were found consistently resistant to trimethoprim in vitro. Therefore, when infection with Moraxella catarrhalis is suspected, the use of alternative antimicrobial agents should be considered. PRIMSOL is not indicated for prophylactic or prolonged administration in otitis media at any age.
Adults
Urinary Tract Infections
For the treatment of initial episodes of uncomplicated urinary tract infections due to susceptible strains of the following organisms: Escherichia coli, Proteus mirabilis, Klebsiella pneumoniae, Enterobacter species and coagulase-negative Staphylococcus species, including S. saprophyticus.
Cultures and susceptibility tests should be performed to determine the susceptibility of the bacteria to trimethoprim. Therapy may be initiated prior to obtaining the results of these tests.
CLINICAL STUDIES
The results of one multicenter, 30-day, comparative, randomized clinical trial without tympanocentesis in 262 pediatric patients with acute otitis media (AOM) are shown below. In this clinical trial, strict evaluability criteria were used to determine clinical response.
| PRIMSOL | SMX + TMP* | |
|---|---|---|
|
||
| Enrolled | 133 | 129 |
| Evaluable | 130 | 129 |
| Clinical Cure | 64/130 (49%) | 63/129 (49%) |
| Clinical Improvement | 30/130 (23%) | 31/129 (24%) |
| Relapse/Recurrence | 19/130 (15%) | 18/129 (14%) |
| Outcome (based on 95% confidence interval) | PRIMSOL equivalent to TMP + SMX | |
The results of an uncontrolled 30-day trial with tympanocentesis in 120 pediatric patients with AOM are shown below:
| Number of patients | ||
|---|---|---|
| Enrolled | 120 | |
| Clinically Evaluable | 102 | |
| Microbiologically Evaluable | 58 | |
| Clinical Cure | 50/102 (49%) | |
| Clinical Improvement | 22/102 (22%) | |
| Clinical Relapse/Recurrence | 20/102 (20%) | |
| Microbiologic Eradication Rates n=58 | Day 5 post-therapy | Day 20 post-therapy |
| Streptococcus pneumoniae | 16/20 (80%) | 14/20 (70%) |
| Haemophilus influenzae | 14/17 (82%) | 13/17 (77%) |
Moraxella catarrhalis, isolated from five patients, was found consistently resistant to trimethoprim in vitro.
CONTRAINDICATIONS
PRIMSOL is contraindicated in individuals hypersensitive to trimethoprim and in those with documented megaloblastic anemia due to folate deficiency.
WARNINGS
Experience with trimethoprim alone is limited, but it has been reported rarely to interfere with hematopoiesis, especially when administered in large doses and/or for prolonged periods.
The presence of clinical signs such as sore throat, fever, pallor or purpura may be early indications of serious blood disorders.
PRECAUTIONS
General
Trimethoprim should be given with caution to patients with possible folate deficiency. Folates may be administered concomitantly without interfering with the antibacterial action of trimethoprim. Trimethoprim should also be given with caution to patients with impaired renal or hepatic function. If any clinical signs of a blood disorder are noted in a patient receiving trimethoprim, a complete blood count should be obtained and the drug discontinued if a significant reduction in the count of any formed blood element is found.
Drug Interactions
PRIMSOL may inhibit the hepatic metabolism of phenytoin. Trimethoprim, given at a common clinical dosage, increased the phenytoin half-life by 51% and decreased the phenytoin metabolic clearance rate by 30%. When administering these drugs concurrently, one should be alert for possible excessive phenytoin effect.
Drug/Laboratory Test Interactions
Trimethoprim can interfere with a serum methotrexate assay as determined by the competitive binding protein technique (CBPA) when a bacterial dihydrofolate reductase is used as the binding protein. No interference occurs, however, if methotrexate is measured by a radioimmunoassay (RIA).
The presence of trimethoprim may also interfere with the Jaffé alkaline picrate reaction assay for creatinine resulting in overestimations of about 10% in the range of normal values.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals to evaluate carcinogenic potential have not been conducted with trimethoprim. Trimethoprim was demonstrated to be non-mutagenic in the Ames assay. No chromosomal damage was observed in human leukocytes cultured in vitro with trimethoprim; the concentration used exceeded blood levels following therapy with PRIMSOL. No adverse effects on fertility or general reproductive performance were observed in rats given trimethoprim in oral dosages as high as 70 mg/kg/day for males and 14 mg/kg/day for females.
Pregnancy
Teratogenic Effects
Pregnancy Category C
Trimethoprim has been shown to be teratogenic in the rat when given in doses 40 times the human dose. In some rabbit studies, the overall increase in fetal loss (dead and resorbed and malformed conceptuses) was associated with doses 6 times the human therapeutic dose.
While there are no large well-controlled studies on the use of trimethoprim in pregnant women, Brumfitt and Pursell,3 in a retrospective study, reported the outcome of 186 pregnancies during which the mother received either placebo or trimethoprim in combination with sulfamethoxazole. The incidence of congenital abnormalities was 4.5% (3 of 66) in those who received placebo and 3.3% (4 of 120) in those receiving trimethoprim plus sulfamethoxazole.
There were no abnormalities in the 10 children whose mothers received the drug during the first trimester. In a separate survey, Brumfitt and Pursell also found no congenital abnormalities in 35 children whose mothers had received trimethoprim plus sulfamethoxazole at the time of conception or shortly thereafter.
Because trimethoprim may interfere with folic acid metabolism, PRIMSOL should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
ADVERSE REACTIONS
To report SUSPECTED ADVERSE REACTIONS, contact FSC Laboratories, Inc. at 1-866-764-7822, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Adverse Events Reported During Pediatric Clinical Trials With PRIMSOL
The following table lists those drug-related adverse events reported most frequently during the clinical trials in pediatric patients aged 6 months to 12 years. Most of these events were determined to be mild. The incidence of drug-related adverse events was significantly lower for PRIMSOL, which was most apparent for those events related to skin/appendages as a body system.
| Drug-related Adverse Event | Percent of Pediatric Patients | |
|---|---|---|
| PRIMSOL (N=310) | SMX + TMP*
(N=197) |
|
|
||
| Body as a whole abdominal pain | <1 | 2.5 |
| Digestive system diarrhea vomiting | 4.2 1.6 | 4.6 1.5 |
| Skin/Appendages rash | 1.3 | 6.1 |
An increase in lymphocytes and eosinophils was noted in some pediatric patients following treatment with PRIMSOL or sulfamethoxazole + trimethoprim oral suspension.
Adverse Reactions Reported For Trimethoprim
In addition to the adverse events listed above which have been observed in pediatric patients receiving PRIMSOL, the following adverse reactions and altered laboratory tests have been previously reported for trimethoprim and therefore, may occur with PRIMSOL therapy:
Dermatologic reactions: pruritus and exfoliative dermatitis. At the recommended adult dosage regimens of 100 mg b.i.d., or 200 mg q.d., each for 10 days, the incidence of rash is 2.9% to 6.7%. In clinical studies which employed high doses of trimethoprim in adults, an elevated incidence of rash was noted. These rashes were maculopapular, morbilliform, pruritic and generally mild to moderate, appearing 7 to 14 days after the initiation of therapy.
Gastrointestinal reactions: Epigastric distress, nausea, and glossitis.
Hematologic reactions: Thrombocytopenia, leukopenia, neutropenia, megaloblastic anemia and methemoglobinemia.
Metabolic reactions: Hyperkalemia, hyponatremia.
Miscellaneous reactions: Fever, elevation of serum transaminase and bilirubin, and increases in BUN and serum creatinine levels.
OVERDOSAGE
Acute
Signs of acute overdosage with trimethoprim may appear following ingestion of 1 gram or more of the drug and include nausea, vomiting, dizziness, headaches, mental depression, confusion and bone marrow depression (see OVERDOSAGE-Chronic).
Treatment consists of gastric lavage and general supportive measures. Acidification of the urine will increase renal elimination of trimethoprim. Peritoneal dialysis is not effective and hemodialysis only moderately effective in eliminating the drug.
Chronic
Use of trimethoprim at high doses and/or for extended periods of time may cause bone marrow depression manifested as thrombocytopenia, leukopenia and/or megaloblastic anemia. If signs of bone marrow depression occur, trimethoprim should be discontinued and the patient should be given leucovorin, 3 to 6 mg intramuscularly daily for three days, or as required to restore normal hematopoiesis.
DOSAGE AND ADMINISTRATION
Acute Otitis Media in Pediatric Patients
The recommended dose for pediatric patients with acute otitis media is 10 mg/kg trimethoprim per 24 hours, given in divided doses every 12 hours for 10 days. The following table is a guideline for the attainment of this dosage:
| Weight | Dose (every 12 hours) | ||
|---|---|---|---|
| lb | kg | tsp | mL |
| 11 | 5 | ½ | 2.5 |
| 22 | 10 | 1 | 5 |
| 33 | 15 | 1½ | 7.5 |
| 44 | 20 | 2 | 10 |
| 55 | 25 | 2½ | 12.5 |
| 66 | 30 | 3 | 15 |
| 77 | 35 | 3½ | 17.5 |
| ≥88 | ≥40 | 4 | 20 |
HOW SUPPLIED
PRIMSOL (trimethoprim hydrochloride oral solution) is a dye-free, alcohol-free, bubble gum flavored, oral solution containing trimethoprim hydrochloride equivalent to 50 mg of trimethoprim in each 5 mL.
NDC 13551-501-01: 20 mL (3/4 ounce)
NDC 13551-501-05: 473 mL (1 Pint)
REFERENCES
- 1
- National Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically - -Third Edition. Approved Standard NCCLS Document M7-A3, Vol. 13, No. 25, NCCLS, Villanova, PA, December, 1993.
- 2
- National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Disk Susceptibility Tests - Fifth Edition. Approved Standard NCCLS Document M2-A5, Vol. 13, No. 24, NCCLS, Villanova, PA, December, 1993.
- 3
- Brumfitt W, Pursell R: Trimethoprim/Sulfamethoxazole in the Treatment of Bacteriuria in Women, J Infect Dis 128 (suppl): S657-S663, 1973.
| PRIMSOL
trimethoprim hydrochloride solution |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - Avadel CNS Pharmaceuticals, LLC (117441358) |
