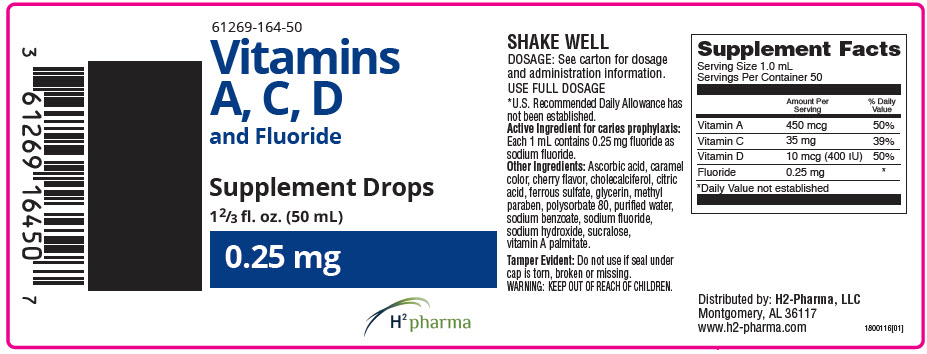
Label: VITAMINS A, C, D AND FLUORIDE DROPS- vitamin a palmitate, ascorbic acid, cholecalciferol, and sodium fluoride solution/ drops
- NHRIC Code(s): 61269-164-50
- Packager: H2-Pharma, LLC
- Category: DIETARY SUPPLEMENT
- DEA Schedule: None
- Marketing Status: Dietary Supplement
Drug Label Information
Updated January 24, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
STATEMENT OF IDENTITY
Supplement Facts Serving Size 1.0 mL Servings Per Container 50 Amount Per Serving % Daily Value - *
- Daily Value not established
Vitamin A 1500 IU 60% Vitamin C 35 mg 88% Vitamin D 400 IU 100% Fluoride 0.25 mg * Active ingredient for caries prophylaxis: Fluoride as sodium fluoride.
Other ingredients: Ascorbic acid, caramel color, cholecalciferol, citric acid, ferrous sulfate, flavor, glycerin, methyl paraben, polysorbate 80, purified water, sodium benzoate, sodium hydroxide, sodium fluoride, sucralose, vitamin A palmitate.
-
CLINICAL PHARMACOLOGY
It is well established that fluoridation of the water supply (1 ppm fluoride) during the period of tooth development leads to a significant decrease in the incidence of dental caries. Hydroxyapatite is the principal crystal for all calcified tissue in the human body. The fluoride ion reacts with the hydroxyapatite in the tooth as it is formed to produce the more caries-resistant crystal, fluorapatite. The reaction may be expressed by the equation:
Ca10(PO4)6(OH)2+2F− -> Ca10(PO4)6F2+2OH− (Hydroxyapatite) (Fluorapatite) Three stages of fluoride deposition in tooth enamel can be distinguished: 1. Small amounts (reflecting the low levels of the fluoride in tissue fluids) are incorporated into the enamel crystals while they are being formed. 2. After enamel has been laid down, fluoride deposition continues in the surface enamel. Diffusion of fluoride from the surface inward is apparently restricted. 3. After eruption, the surface enamel acquires fluoride from water, food, supplementary fluoride and smaller amounts from salvia.
Supplementation of the diet with Vitamins A, C, D and Fluoride 0.25 mg Drops also provides fluoride for caries prophylaxis. The American Academy of Pediatrics recommends that children up to age 16, in areas where drinking water contains less than optimal levels of fluoride, receive daily fluoride supplementation. Vitamins A, C, D and Fluoride 0.25 mg Drops provide fluoride in drop form for infants and young children 6 months to 3 years of age, in areas where the drinking water contains less than 0.3 ppm of fluoride, and for children ages 3-6 years, in areas where the drinking water contains 0.3 through 0.6 ppm of fluoride. Each 1.0 mL supplies sodium fluoride (0.25 mg fluoride) plus three basic vitamins. The American Academy of Pediatrics recommends that infants and young children 6 months to 3 years of age, in areas where the drinking water contains less than 0.3 ppm of fluoride; and for children ages 3-6 years, in areas where the drinking water contains 0.3 through 0.6 ppm of fluoride, receive 0.25 mg of supplemental fluoride daily which is provided in a full dose (1 mL) of Vitamins A, C, D and Fluoride 0.25 mg Drops. A comprehensive 5 ½ year series of studies of the effectiveness of multivitamins and fluoride drops in caries protection has been published. Children in this continuing study lived in an area where the water supply contained only 0.05 ppm fluoride. The subjects were divided into two groups: one which used only the non-fluoride vitamin drops, and the other group used multivitamin-fluoride drops. The three year interim report showed 63% fewer carious surfaces in primary teeth and 43% fewer carious surfaces in permanent teeth of the children taking multivitamin-fluoride products. After four years, the studies continued to support the effectiveness of multivitamin-fluoride products, showing a reduction in carious surfaces of 68% in primary teeth and 46% in permanent teeth. Results at the end of the 5 ½ years further confirmed the previous findings and indicated that significant reductions in dental caries are apparent with the continued use of multivitamin-fluoride products. *These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.
- WARNINGS
-
PRECAUTIONS
The suggested dose should not be exceeded since dental fluorosis may result from continued ingestion of large amounts of fluoride. When recommending vitamin fluoride products: 1. Determine the fluoride content of the drinking water. 2. Make sure the child is not receiving significant amounts of fluoride from other medications and swallowed toothpaste. 3. Periodically check to make sure that the child does not develop significant dental fluorosis. 4. Vitamins A, C, D and Fluoride Supplemental Drops 0.25 mg should be dispensed in the original plastic container, since contact with glass leads to instability and precipitation. (The amount of sodium fluoride in the 50 mL size is well below the maximum to be dispensed at one time according to recommendations of the American Dental Association.)
- ADVERSE REACTIONS
- DOSAGE AND ADMINISTATION
- HOW SUPPLIED
- RECOMMENDED STORAGE
- HEALTH CLAIM
- PRINCIPAL DISPLAY PANEL - 50 mL Bottle Label
-
INGREDIENTS AND APPEARANCE
VITAMINS A, C, D AND FLUORIDE DROPS
vitamin a palmitate, ascorbic acid, cholecalciferol, and sodium fluoride solution/ dropsProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:61269-164 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) (VITAMIN A - UNII:81G40H8B0T) VITAMIN A 450 ug in 1 mL ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 35 mg in 1 mL CHOLECALCIFEROL (UNII: 1C6V77QF41) (CHOLECALCIFEROL - UNII:1C6V77QF41) CHOLECALCIFEROL 10 ug in 1 mL SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 0.25 mg in 1 mL Inactive Ingredients Ingredient Name Strength CARAMEL (UNII: T9D99G2B1R) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) FERROUS SULFATE (UNII: 39R4TAN1VT) CHERRY (UNII: BUC5I9595W) GLYCERIN (UNII: PDC6A3C0OX) METHYLPARABEN (UNII: A2I8C7HI9T) POLYSORBATE 80 (UNII: 6OZP39ZG8H) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM HYDROXIDE (UNII: 55X04QC32I) SUCRALOSE (UNII: 96K6UQ3ZD4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:61269-164-50 1 in 1 CARTON 1 50 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date DIETARY SUPPLEMENT 01/04/2017 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color flavor Labeler - H2-Pharma, LLC (028473634)