Label: COLACE- docusate sodium - sennosides tablet, film coated
- NDC Code(s): 67618-110-10, 67618-110-30, 67618-110-60
- Packager: Atlantis Consumer Healthcare, Inc.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated December 18, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
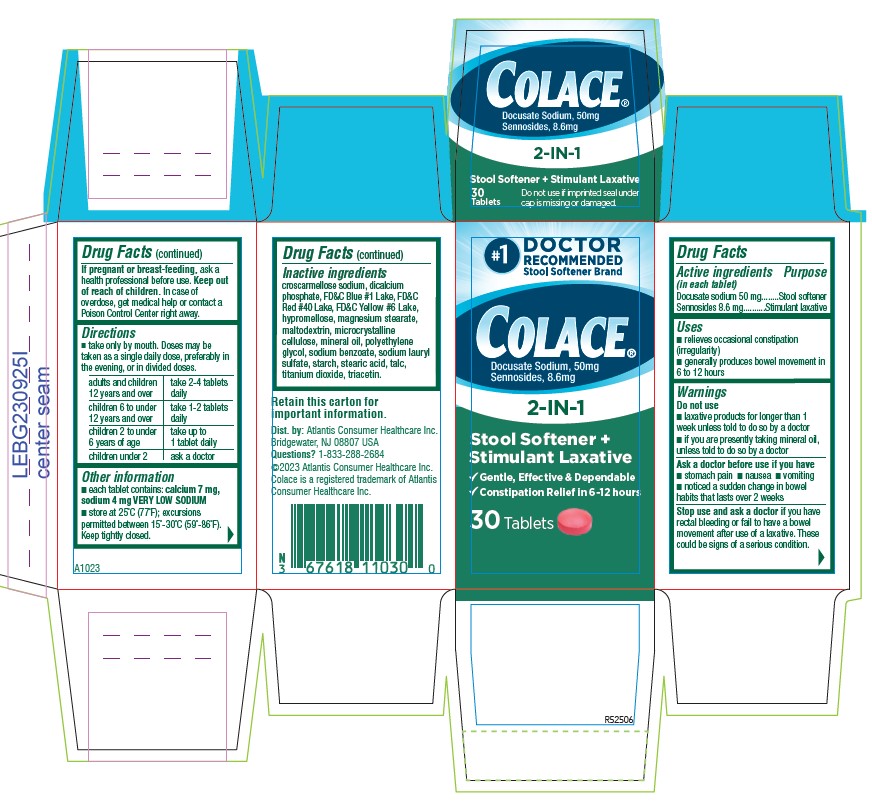
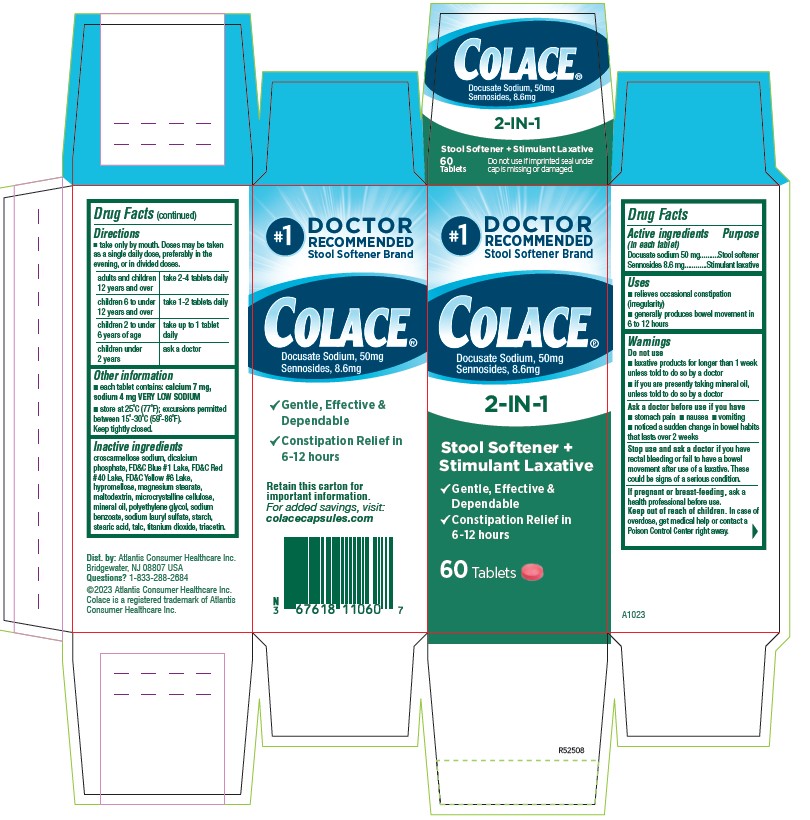
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- ASK DOCTOR
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
- Take only by mouth. Doses may be taken as a single daily dose, preferably in the evening, or in divided doses.
adults and children 12 years and over take 2-4 tablets daily children 6 to under 12 years of age take 1-2 tablets daily children 2 to under 6 years of age take up to 1 tablet daily children under 2 ask a doctor - HOW SUPPLIED
-
INACTIVE INGREDIENT
croscarmellose sodium, dicalcium phosphate, FD&C Blue #1 Lake, FD&C Red #40 Lake, FD&C Yellow #6 Lake, hypromellose, magnesium stearate, maltodextrin, microcrystalline cellulose, mineral oil, polyethylene glycol, sodium benzoate, sodium lauryl sulfate, starch, stearic acid, talc, titanium dioxide, triacetin.
Dist. by: Atlantis Consumer Healthcare inc.
Bridgewater, NJ 08801 USA
Questions? 1-833-286-2684
©2023 Atlantis Consumer Healthcare inc.
Colace is a registered trademark of Atlantis
Consumer Healthcare Inc.
A1023
R52504 -
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
#1
DOCTOR
RECOMMENDED
Stool Softener Brand
Colace®
Docusate Sodium, 50 mg
Sennosides 8.6 mg
2-IN-1
Stool Softener +
Stimulant Laxative
√ Gentle, Effective, & Dependable
√ Constipation Relief in 6 `12 hours
10 Tablets
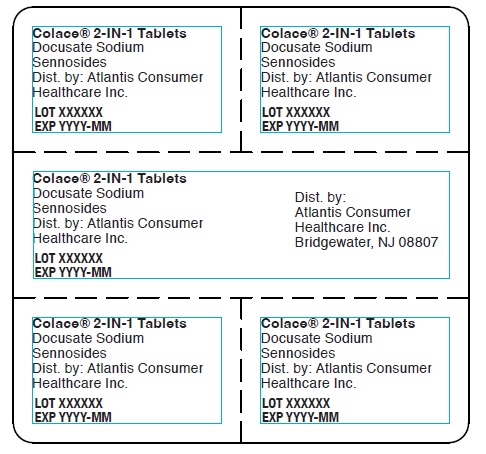
Colace® 2-IN-1 Tablets
Docusate Sodium
Sennosides
Dist. by: Atlantis Consumer
Healthcare Inc.
LOT XXXXXX
EXP YYYY-MM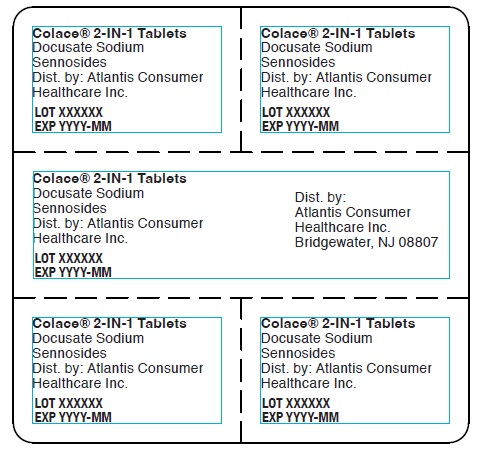
#1
DOCTOR
RECOMMENDED
Stool Softener Brand
Colace®
Docusate Sodium, 50 mg
Sennosides 8.6 mg
2-IN-1
Stool Softener +
Stimulant Laxative
√ Gentle, Effective, & Dependable
√ Constipation Relief in 6 `12 hours
30 Tablets
Retain this carton for
Important information
Dist. by: Atlantis Consumer Healthcare inc.
Bridgewater, NJ 08801 USA
Questions? 1-833-286-2684
©2023 Atlantis Consumer Healthcare inc.
Colace is a registered trademark of Atlantis
Consumer Healthcare Inc.
A1023
R52506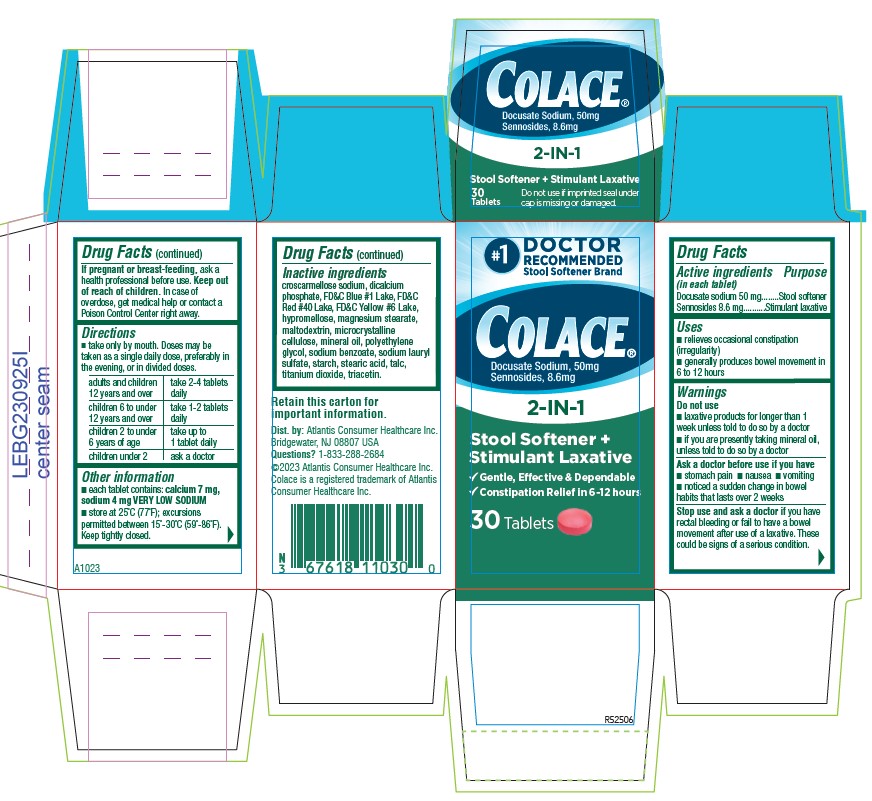
Colace®
Docusate Sodium
Sennosides
2-IN-1
Stool Softener +
Stimulant Laxative
30 Tablets
Do not use if imprinted seal under cap
is missing or damaged.
See carton for additional information
Dist. by: Atlantis Consumer Healthcare inc.
Bridgewater, NJ 08801 USA
Questions? 1-833-286-2684
©2023 Atlantis Consumer Healthcare inc.
Colace is a registered trademark of Atlantis
Consumer Healthcare Inc.
A1023
R52507
#1
DOCTOR
RECOMMENDED
Stool Softener Brand
Colace®
Docusate Sodium, 50 mg
Sennosides 8.6 mg
2-IN-1
Stool Softener +
Stimulant Laxative
√ Gentle, Effective, & Dependable
√ Constipation Relief in 6 `12 hours
Retain this carton for
Important information
For added savings, visit:
colacecapsules.com
Dist. by: Atlantis Consumer Healthcare inc.
Bridgewater, NJ 08801 USA
Questions? 1-833-286-2684
©2023 Atlantis Consumer Healthcare inc.
Colace is a registered trademark of Atlantis
Consumer Healthcare Inc.
A1023
R52508
60 Tablets
A1023
R52507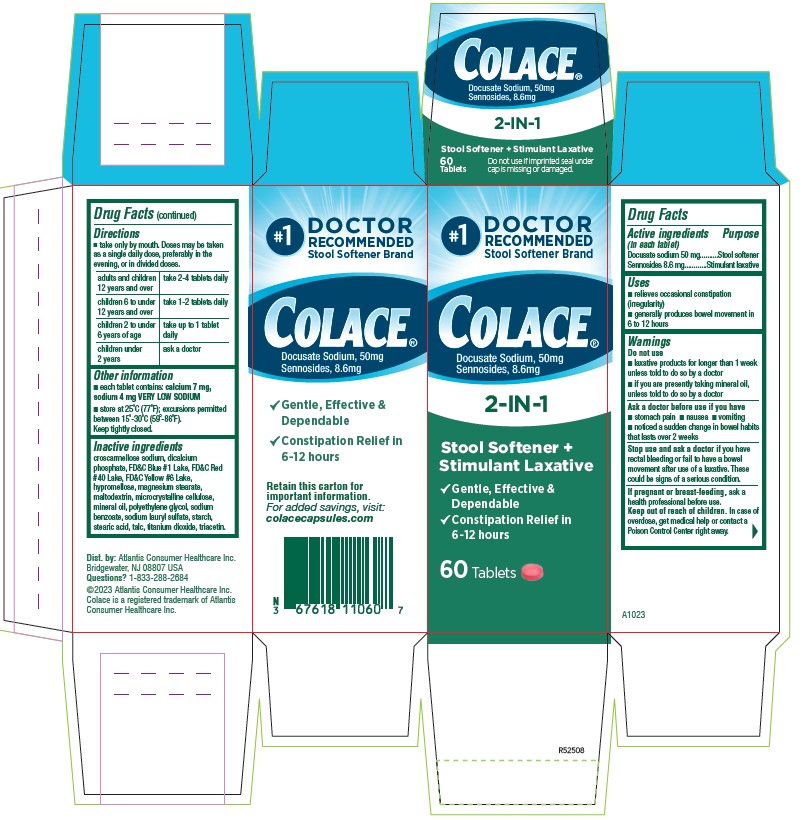
Colace®
Docusate Sodium
Sennosides
2-IN-1
Stool Softener +
Stimulant Laxative
60 Tablets
Do not use if imprinted seal under cap
is missing or damaged.
See carton for additional information
Dist. by: Atlantis Consumer Healthcare inc.
Bridgewater, NJ 08801 USA
Questions? 1-833-286-2684
©2023 Atlantis Consumer Healthcare inc.
Colace is a registered trademark of Atlantis
Consumer Healthcare Inc.
LOT:
EXP:
A1023
R52509
-
INGREDIENTS AND APPEARANCE
COLACE
docusate sodium - sennosides tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:67618-110 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOCUSATE SODIUM (UNII: F05Q2T2JA0) (DOCUSATE - UNII:M7P27195AG) DOCUSATE SODIUM 50 mg SENNOSIDES (UNII: 3FYP5M0IJX) (SENNOSIDES - UNII:3FYP5M0IJX) SENNOSIDES 8.6 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) MICROCRYSTALLINE CELLULOSE 102 (UNII: PNR0YF693Y) MINERAL OIL (UNII: T5L8T28FGP) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM LAURYL SULFATE (UNII: 368GB5141J) STARCH, CORN (UNII: O8232NY3SJ) STEARIC ACID (UNII: 4ELV7Z65AP) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) Product Characteristics Color RED (burgundy) Score no score Shape ROUND Size 10mm Flavor Imprint Code P;054 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67618-110-10 2 in 1 CARTON 02/08/1957 1 5 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:67618-110-30 1 in 1 CARTON 02/08/1957 2 30 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 3 NDC:67618-110-60 1 in 1 CARTON 02/08/1957 3 60 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M007 02/08/1957 Labeler - Atlantis Consumer Healthcare, Inc. (118983925)