PENNCHLOR S 40/40- chlortetracycline calcium and sulfamethazine powder
Pharmgate Animal Health
----------
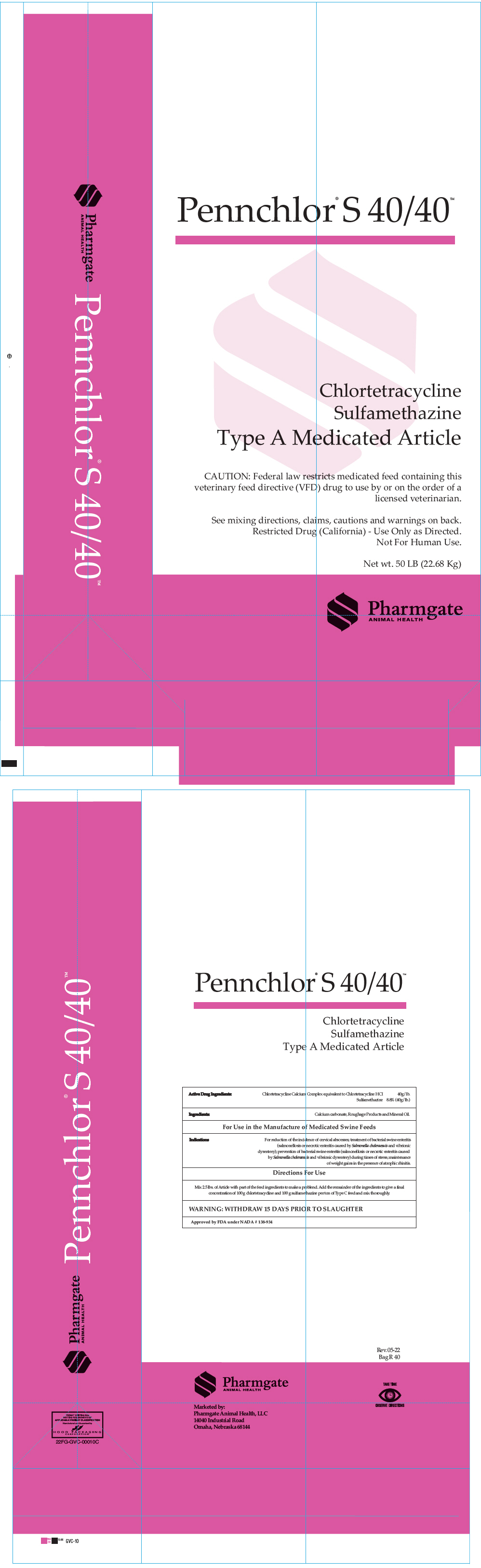
Pennchlor® S 40/40™
| Active Drug Ingredients: | Chlortetracycline calcium complex equivalent to Chlortetracycline HCl | 40 g/lb. | |
| Sulfamethazine | 8.8% (40 g/lb.) | ||
| Ingredients: Calcium carbonate, Roughage Products and Mineral Oil | |||
| For Use in the Manufacture of Medicated Swine Feeds | |||
| Indications | For reduction of the incidence of cervical abscesses; treatment of bacterial swine enteritis (salmonellosis or necrotic enteritis caused by Salmonella choleraesuis and vibrionic dysentery); prevention of bacterial swine enteritis (salmonellosis or necrotic enteritis caused by Salmonella choleraesuis and vibrionic dysentery) during times of stress; maintenance of weight gains in the presence of atrophic rhinitis. | ||
| Directions For Use | |||
| Mix 2.5 lbs. of Article with part of the feed ingredients to make a preblend. Add the remainder of the ingredients to give a final concentration of 100g chlortetracycline, and 100g sulfamethazine per ton of Type C feed and mix thoroughly. | |||
| WARNING: WITHDRAW 15 DAYS PRIOR TO SLAUGHTER | |||
| Approved by FDA under NADA # 138-934 | |||
Marketed by:
PHARMGATE ANIMAL HEALTH, LLC
14040 Industrial Road
Omaha, Nebraska 68144
Take Time Observe Directions
Observe Directions
PRINCIPAL DISPLAY PANEL - 22.68 Kg Bag
Pennchlor® S 40/40™
Chlortetracycline
Sulfamethazine
Type A Medicated Article
CAUTION: Federal law restricts medicated feed containing this
veterinary feed directive (VFD) drug to use by or on the order of a
licensed veterinarian.
See mixing directions, claims, cautions and warnings on back.
Restricted Drug (California) - Use Only as Directed.
Not For Human Use.
Net wt. 50 LB (22.68 Kg)
Pharmgate
ANIMAL HEALTH
| PENNCHLOR S 40/40
chlortetracycline calcium and sulfamethazine powder |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Pharmgate Animal Health (833270817) |
| Registrant - Pharmgate Inc. (079628671) |
Revised: 6/2023
Document Id: 43feb5a4-40ae-4c41-8b7f-0d8d56ad3d9d
Set id: 9f789b37-ba94-4f5b-a130-b0ecfb2d48b0
Version: 5
Effective Time: 20230607
Pharmgate Animal Health